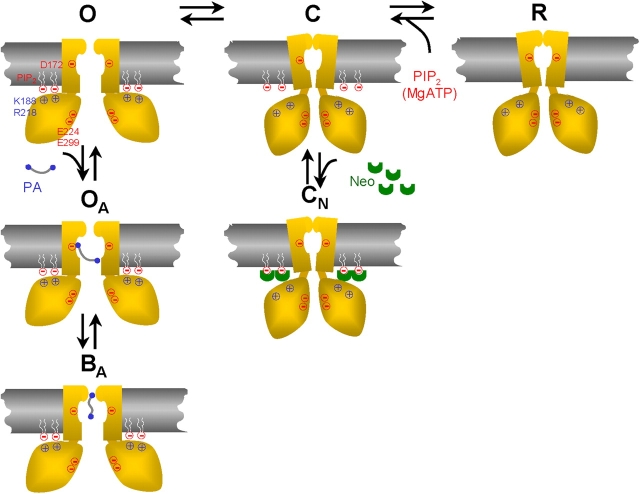
Figure 7.
Schematic model of modulation of Kir channels by PIP2 and long polyamines. Only two M2 helices and two cytoplasmic regions of the tetrameric structure are shown for clarity. The channel opens (O state) when the conserved positively charged residues (e.g., K188, R218) in the cytoplasmic region bind to negatively charged heads of PIP2 molecules, and closes (C) when this interaction is lost. The Kir2.1 has a high open probability of >0.9, suggesting a normally high PIP2 binding affinity. When PIP2 is hydrolyzed by PLC or screened by neomycin (Neo), the channel closes (CN state). The absence of new PIP2 to interact causes channel rundown (R state). Long polyamines (PA) interact with D172 in the M2 region to allosterically strengthen the interaction of the cytoplasmic domain with PIP2, thereby locking the channel in the open configuration (OA state) and preventing rundown or inhibition by neomycin. The channel is blocked at positive membrane potential by polyamine plugging the pore near the selectivity filter (BA state).