Abstract
Tendons and ligaments are elastic collagenous tissues with similar composition and hierarchical structure, contributing to motion. Their strength is related to the number and size of the collagen fibrils. Collagen fibrils increase in size during development and in response to increased physical demands or training. Tendon disorders are commonly seen in clinical practice and give rise to significant morbidity. Treatment is difficult and patients often suffer from the symptoms for quite a long time. Despite remodelling, the biochemical and mechanical properties of healed tendon tissue never match those of intact tendon. The prerequisite for focussed treatment strategies in the future will be an improved understanding of the molecular events both in the embryo and contributing to regeneration in the adult organism. Novel approaches include the local delivery of growth factors, stem- and tendon-cell-derived therapy, the application of mechanical load and gene-therapeutic approaches based on vehicles encoding selected factors, or combinations of these. Important factors are proteins of the extracellular matrix like the metalloproteinases, growth factors like the bone morphogenetic proteins but also intracellular signalling mediator proteins, such as the Smads and transcription factors from the helix–loop–helix and other families. In this review, we focus specifically on such molecular approaches based on mesenchymal stem cells.
Résumé
Les tendons et les ligaments sont constitués de fibres élastiques de collagène dont la composition est similaire de même que leur structure contribuant au mouvement. Leur résistance est parallèle au nombre et à la taille des fibres collagènes. Si les fibres collagènes grossissent durant la croissance, il en est de même en réponse à une augmentation de l’entraînement physique. A titre clinique on rencontre relativement fréquemment les problèmes tendineux responsables d’une certaine morbidité. Le traitement en est difficile, les patients sont affectés sur un temps relativement long de troubles secondaires à ces lésions. En dépit du remodelage, les propriétés biomécaniques et biochimiques d’un tendon, d’un tissu tendineux guéri ne peuvent être comparés à ceux d’un tendon sain. Les prérequis d’une stratégie thérapeutique devrait, dans le futur, permettre de mieux comprendre ce qui se passe au moment du développement embryologique et de la régénération au niveau de l’organisme adulte. Une nouvelle approche thérapeutique doit prendre en compte l’administration de facteurs de croissance et l’utilisation de cellules souche dans le cadre d’une thérapie génique. Les facteurs importants sont les protéines de la matrice extracellulaire comme les métalloprotéinases de même que les facteurs de croissance de type BMP mais il faut prendre en compte également les facteurs de transcriptions chromosomiques. Pour cette étude, nous nous sommes spécialement centrés sur de telles molécules et sur les cellules souches mesenchymenteuses.
Structure and composition of tendons and ligaments
Ligaments connect bones to each other in order to restrict their relative motions. According to this definition, the patellar tendon in fact is a ligament. Ligaments are pliable but essentially not elastic, contrasting with tendons. Tendons link muscles to bone at the musculo-tendinous junction and osteo-tendinous junction or enthesis, respectively. The structure and composition of tendons and ligaments are similar: water is predominant at 55–70% (higher in tendons). Additional components are collagen (type I predominant, some type III) amounting to 70–80% of the dry weight, elastin, proteoglycans, glycosaminoglycans and glycoproteins (e.g. fibronectin, thrombospondin). Fibrillar collagen type I gives ligaments and tendons their high tensile strength and is responsible for the hierarchical structure (Fig. 1): soluble tropocollagen molecules (a triple helix with two alpha-1 chains and one alpha-2 chain) spontaneously self-assemble after secretion and cross-linking (which results in the formation of insoluble collagen molecules) into collagen microfibrils. Microfibrils arrange themselves into larger units, called subfibrils, in ligaments or subfascicles/primary fibre bundles in tendons. The fibrils then gather into collagen fibres or fascicles/secondary fibre bundles. In addition, tendons are bound together by the endotenon, a loose connective tissue that also includes blood, lymph vessels and nerves and is continuous with the epitenon which surrounds the whole tendon. Surrounding the epitenon superficially, another thin layer, called paratenon, is present, which allows free movements within the surrounding tissue. Epi- and paratenon together constitute the peritenon. Long tendons such as the digital flexor tendon are additionally enclosed in a synovial sheath that gives lubrication and enhances gliding.
Fig. 1.
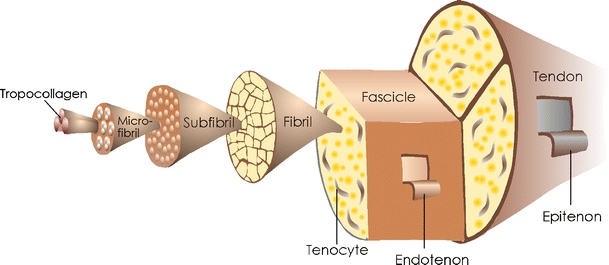
The organisational structure of tendons
The cellular elements within ligaments and tendons—fibroblasts that synthesise and secrete the collagen and all components of the extracellular matrix—are interspersed between the collagen bundles and lie along the long axis. The fibroblasts are termed “tenoblasts” or “ligamentoblasts” when still immature. They are spindle-shaped. As they age, teno-/ligamentoblasts become elongated and transform into “teno-/ligamentocytes.” Together, tenoblasts and tenocytes account for 90–95% of the cellular elements of tendons. The remaining 5–10% consists of chondrocytes at the bone attachment and insertion sites, synovial cells of the tendon sheath and vascular cells, including capillary endothelial cells and smooth muscle cells of arterioles [39].
Properties and functions
Ligaments and tendons are extraordinarily strong in resisting tensile loads. For example, the digital flexor tendon of a horse is strong enough to support the weight of two large automobiles [28]. Tendons are viscoelastic tissues in exhibiting time-dependent strain and relaxation rates. Below a strain of about 4%, a tendon behaves in an elastic way, which means that it returns to its original length and performance upon strain release. A strain in excess of 4% causes microscopic or macroscopic failure [22].
A second and less often mentioned function of tendinous and ligamentous tissue is proprioception: mechanoreceptors serve to protect, e.g. the knee, from extremes of motion, thereby, maintaining the stability of joints or provide feedback control that changes muscle activity when resistance to movement is encountered.
Tendons and ligaments consume comparatively low amounts of energy by themselves, resulting in a modest metabolic rate. Concomitant to their well-developed anaerobic energy generation (glycolysis and pentose phosphate cycle as opposed to the aerobic Krebs or citrate cycle), this enables them to bear loads and maintain tension for extended periods of time. This, in turn, reduces the incidence of ischemia and, consequently, necrosis. On the other hand, the low metabolic rate entails slow healing after injury, as will be discussed below.
Pathology and healing
Tendon and ligament injuries account for considerable morbidity and often prove disabling for several months. They can be acute or chronic and are caused by intrinsic and/or extrinsic factors. Intrinsic factors are: age, gender, biomechanics, systemic diseases like rheumatoid arthritis and probably genetic factors as discussed by Rees et al. [35]. They can be manipulated only marginally. Extrinsic factors include: physical load, environment, occupation and training. There are different terms for tendon injuries: the term “tendinosis” describes a degenerative tendon without accompanying inflammation, while “tendinopathy” depicts an inflammatory reaction subsequent to rupture or vascular damage [35]. “Tenosynovitis” implies inflammatory changes in synovial sheaths and is a term commonly used in any tendon sheath disorder [6].
Tendon healing occurs in three overlapping phases [40]: first, the inflammatory stage lasting hours to a few days; second, the remodelling stage with collagen type III synthesis; and third, the modelling stage after about 6 weeks, which can be divided into a consolidation and a final maturation phase, during which, the healing tissue is resized and reshaped. During the consolidation phase, collagen type I synthesis is initiated. In the maturation stage, the fibrous tissue slowly (within several months) transforms into scar tissue.
Molecular players in tendon formation and regeneration
The prerequisite for focussed treatment strategies in the future requires an improved understanding of the molecular events leading to tendon/ligament formation in the embryo on one side and contributing to regeneration in the adult organism on the other. During embryogenesis, a unique compartment of the somite, called the syndetome, provides tenocyte progenitors for the development of the axial tendons. Inductive interactions between the well-described myotome and sclerotome layers generate the syndetome, demarcated at the earliest stages of development by the expression of the basic helix–loop–helix (HLH) transcription factor Scleraxis (Scx) [8]. A comparable system for the development of appendicular tendons may be envisaged. However, although Scx expression has been implicated as necessary, it seems not sufficient to trigger tendon and ligament formation: in C3H10T1/2 mesenchymal progenitor cells, the overexpression of Scx in vitro is not sufficient for tenocyte formation (own observations) and also retroviral misexpression in the chick limb bud did not exhibit a significant phenotype [37]. Although it might be noteworthy to remember that tissue-specific HLH transcription factors like Scx work rather like dimers with their ubiquitously expressed partners, the E-proteins and simultaneous overexpression of E-proteins may be required to elicit a significant phenotype. Other transcription factors involved in tendon/ligament formation may be Six1, Six2 (sine oculis; [33]), as well as the Eya proteins, Eya1, Eya2 (eyes absent; [45]), which are expressed during limb formation in developing tendons and ligaments. But, strictly speaking, no master genes and not many real marker genes for tendon or ligament development have yet been identified.
Tendon healing studies have mainly been performed on either transected animal tendons or ruptured human tendons. Due to this fact, their relevance to degenerate human tendons remains unclear. The management of tendon injury still poses a considerable challenge for clinicians: the body is able to heal most areas of damage with fibrous scar tissue with collagen as a major component but the fibres are randomly arranged in all directions. The biochemical and mechanical properties of healed tendon tissue never match those of intact tendon. Therefore, the ultimate aim of all cellular and gene therapies would be to restore functional tissue. In order to pursue this aim, different studies have been undertaken. These include: local delivery of growth factors, stem-cell and tendon-derived cell therapy, application of mechanical load, and gene-therapeutic approaches based on vehicles encoding selected factors, or combinations of these (reviewed, e.g. in [19]).
Local delivery of growth factors
The paracrine signals that fine-tune multipotent mesenchymal progenitors to the unique tenocyte fate during normal development are virtually unknown but they appear to involve FGFs [7] and key members of the transforming growth factor/bone morphogenetic protein superfamily, such as growth and differentiation factor 5 (GDF5), GDF6 and GDF7 [44]. The inactivation of GDF5 and GDF6 genes in mice causes defects in ligaments [10, 29, 38, 41]. The injection of recombinant growth factors or on carriers as supporting materials was performed on injured tendons. GDF5, GDF6 and GDF7 were also successfully used in dose–response studies for rat Achilles tendon healing [12, 13, 42]. Most importantly, however, some of these studies observed not only tendon formation in vivo but also the formation of bone and/or cartilage, resulting in reduced elasticity and stability of the newly formed tendon tissue [1, 44]. Other factors like platelet-derived growth factor [5, 18], epidermal growth factor and insulin-like growth factor [36], alone or in combination, not only directly influence tissue regeneration but are also beneficial in terms of anti-inflammatory function and the chemoattraction of cells. Important issues in all of these approaches are the amount of growth factor administered and the time point of administration, as well as the treatment regimens.
Cell-derived therapies
One possibility is the use of tissue-restricted, in this case, tendon-derived cells. Alternatively, stem cells have the capacity to regenerate tissue (see section titled Genetically modified stem cells: MSCs for gene delivery to tendons) and the influence of the local surroundings may trigger their differentiation into tendinous or ligamentous tissue. Whatever their source, cells are applied to scaffolds and implanted or injected directly into the defect site.
Recently, four cellular sources from different tendon/ligament tissue were investigated for a tissue-engineered ligament replacement [11]. The investigators suggested cells from the anterior cruciate ligament as the most suitable cells for the development of tissue-engineered ligament and tendon.
Strategies for a successful mesenchymal stem cell (MSC) dependent repair of tendon tissue have been described in several studies. MSC–collagen composites were used for tendon repair of the patellar tendon, resulting in a higher capacity to resist maximum stress [2]. A significant part of these MSC–collagen grafted tendons developed bone in the repair site probably due to the high cell to collagen ratios of the implant [23].
Alternatively, many in vitro approaches try to mimic the mechanical and chemical micro-environment that may be necessary to provide tendon-derived cells or MSCs with the cues to develop into tendo-/ligamentocytes. Tendon-derived cells fabricated in a mechanically loaded, linear collagen gel construction assume a phenotype that is similar to that of a native tendon in terms of appearance and expression [14]. MSCs were formatted into a type I collagen gel, the ends fastened to a spring to keep the cells under constant load. The cells were oriented with regard to the suture and aligned with the load axis within an Achilles tendon defect in adult rabbits after implantation. At 3 months, efficient neo-tendon tissue formation was observed in vivo [3, 4, 9, 46]. In conclusion, the mechanical loading of stem cell or tendon-derived cells appears to be a very promising alternative to the classical treatment of tendon disorders but needs clinical evaluation.
Gene-therapeutic strategies
Instead of using recombinant proteins or cells, the delivery of therapeutic genes has been investigated. Two major modalities are available: in vivo local delivery of vehicles (which is favoured in contrast to systemic delivery) or ex vivo modification of cells and their subsequent reinjection or reimplantation. This second approach will be separately discussed in the next section.
Adenovirusses encoding lacZ were injected into rabbit patellar tendon but resulted in only a few cells within the tissue that subsequently actually expressed lacZ [15]. Alternatively, lacZ adenovirusses were transferred into chicken tendon and tendon sheath. Here, tendon staining was found in the epitenon layer and was, therefore, also limited [26]. A third study used lacZ adenovirusses in a model of ruptured versus non-injured medial and anterior cruciate ligaments. In addition, the direct injection of viruses was compared with the injection of virally modified cells. Transfected cells were found incorporated in ligament structures [17]. In conclusion, the direct administration of (adeno)viruses is possible but may not be the optimal choice, since the dense extracellular matrix apparently interferes with efficient infection and also with gene transfer into tenocytes.
Genetically modified stem cells: MSCs for gene delivery to tendons
Adult stem cells open the possibility to treat diverse diseases by autologous transplantation (i.e. using the patient’s own cells after ex vivo expansion), either through local application or through systemic infusion. Alternatively, since human MSCs are thought to be non- or only faintly immunogenic, even the use of allogenic donor cells might become feasible. MSCs have—due to their potential use in regenerative medicine and tissue engineering—obtained high therapeutic relevance.
The implantation of a three-dimensional polycaprolactone fibre scaffold was performed at the knee joint of rabbits to support and deliver lacZ gene-marked allogenic MSCs in combination with a fibrin glue [16]. GDF7-transfected mesenchymal stem cells contributed to the healing of a tendon defect [27].
A novel approach from our group resulted in both ectopic tendon formation and repair of a rat Achilles tendon partial defect [20]: plasmids encoding the growth factor bone morphogenetic protein-2 (BMP2) and a biologically active variant of the Smad8 signalling mediator protein (constitutively active Smad8, Smad8ca) were stably introduced into mesenchymal progenitor cells C3H10T1/2. Smads are a group of related intracellular proteins that transmit TGF-β/BMP-superfamily signals from ligand-activated cell surface receptors to the nucleus (Fig. 2). A constitutively active R-Smad, i.e. a Smad protein that is constantly active, even in the absence of ligand, can be generated artificially. This molecule is also depicted in Fig. 2. In the presence of both BMP2 and Smad8ca, C3H10T1/2 mesenchymal progenitor cells adopt a tenogenic fate as demonstrated in vitro and in vivo on a molecular level, histologically and through double-quantum filtered micro-MRI analysis, which specifically highlights water in oriented collagen bundles. Neither cells expressing Smad8ca alone (in the absence of BMP2), wild-type Smad8 plus BMP2 nor GDF5 or TGF-β are able to give rise to a similar phenotype. Importantly, no bone formation was observed, despite the presence of BMP2.
Fig. 2.
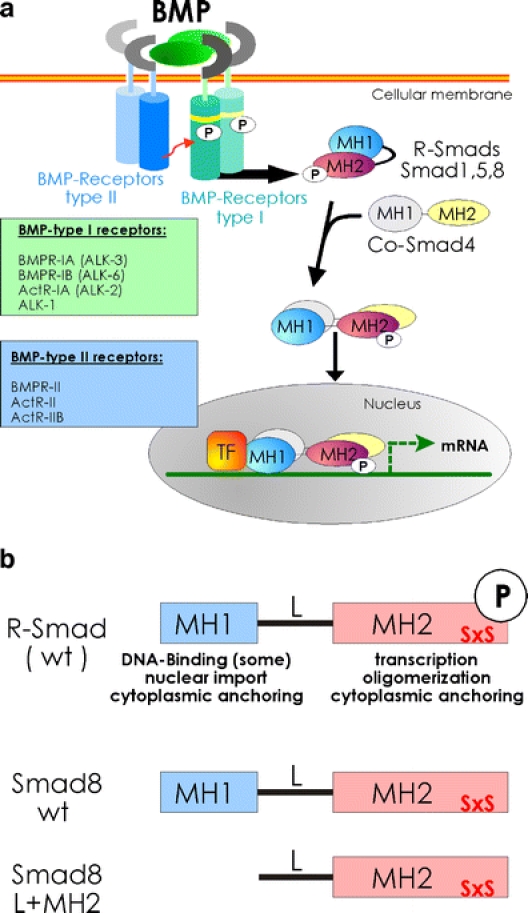
a BMP-mediated Smad-signalling. The constitutively active BMP-type II receptors (blue) phosphorylate the BMP-type I receptors (ALKs; activin-like kinase receptors; green) on specific serine and threonine residues in the GS domain (yellow). These activated BMP-type I receptors propagate the signal downstream by directly phosphorylating the latent and inactive BMP-specific R-Smads Smad1, 5 or 8. The R-Smads form heteromeric complexes with the common mediator Smad4 that translocate into the nucleus. In combination with transcription factors (TF), Smads regulate gene transcription. b R-Smad and L+MH2 structures. The SxS motif located at the distal end at the carboxyterminus of R-Smads is directly phosphorylated by BMP type I receptors to convert the latent Smads to active signalling mediators. Since the Smad8 L+MH2 fragment lacks intrinsic DNA binding, its tenogenic actions are mediated by regulatory protein–protein interactions
What may be the molecular events triggered by the transcription factor Smad8 that ultimately lead to tendon/ligament formation? Our hypothesis is that the highly ordered collagen structure of tendons, which is the principal difference to other connective tissues, is related to the BMP2/Smad8ca signalling system, resulting in the synthesis of secreted matrix components that are necessary and sufficient for the ordered collagen structure and organised extracellular matrix (ECM) micro-environment to form.
The most important system involved in ECM remodelling are the matrix metalloproteinases (MMPs) [34]. In addition, the TIMPs (tissue inhibitor of metalloproteinases) and α2-macroglobulin serve to regulate MMP activity. The interplay between MMPs and TIMPs may be one of the key factors in the generation of the specialised collagen structure of tendons and ligaments [24, 25]. This is in particular highlighted by the activities of MT-1/MMP14. This membrane-bound MMP binds to the tissue inhibitor of metalloproteinase-2 (TIMP2), which binds to pro-MMP2, thereby, positioning it for activation by a second molecule of MMP14 and processing it to active MMP2 [34, 43]. Interestingly, Mmp14 knockout mutants are grossly defective in the remodelling of the connective tissue, show increased bone degradation and also exhibit a tendon phenotype [21]. Based on these observations, one may postulate that a stem-cell-mediated overexpression of, e.g. MT-1/MMP14, in tendons will result in significantly increased tendon remodelling and higher levels of tendon formation.
Moreover, collagen-associated proteins contribute to in vivo collagen fibrillogenesis and regulate the mechanical properties of connective tissues: tenascin-X [30], decorin, biglycan [47] or periostin [32]. Although not explicitly demonstrated in the latter study, periostin may be deeply involved in the structural regulation of collagen fibrillogenesis in tendons. In gene profiling analyses, we could show that periostin is dramatically upregulated in the Smad8-dependent model of tendon formation described previously (unpublished observation). Decorin downregulation exhibited a deep impact on collagen fibrillogenesis and led to a significantly improved healing of tendon defects [31].
Future aspects
The current options for the therapy of tendon and ligament injuries do not provide satisfactory long-term results and new therapeutic modalities are required. New and improved scaffolds have to be developed which exhibit a greater balance between stiffness and elastic compliance. Mechanical stimulation, growth factors and hormones may be needed for an optimal control of the differentiation process during regeneration. The first successful cell and gene therapy approaches involving tendon-derived cells or MSCs for tendon and ligament engineering have been reported and may evolve as an attractive option.
Acknowledgements
The authors gratefully acknowledge the support from the EU integrated project GENOSTEM and by the SFB 599 collaborative research program of the German Research Foundation (DFG).
References
- 1.Aspenberg P, Forslund C (1999) Enhanced tendon healing with GDF 5 and 6. Acta Orthop Scand 70:51–54 [DOI] [PubMed]
- 2.Awad HA, Boivin GP, Dressler MR et al (2003) Repair of patellar tendon injuries using a cell-collagen composite. J Orthop Res 21:420–431 [DOI] [PubMed]
- 3.Awad HA, Butler DL, Boivin GP et al (1999) Autologous mesenchymal stem cell-mediated repair of tendon. Tissue Eng 5:267–277 [DOI] [PubMed]
- 4.Awad HA, Butler DL, Harris MT et al (2000) In vitro characterization of mesenchymal stem cell-seeded collagen scaffolds for tendon repair: effects of initial seeding density on contraction kinetics. J Biomed Mater Res 51:233–240 [DOI] [PubMed]
- 5.Batten ML, Hansen JC, Dahners LE (1996) Influence of dosage and timing of application of platelet-derived growth factor on early healing of the rat medial collateral ligament. J Orthop Res 14:736–741 [DOI] [PubMed]
- 6.Benjamin M, Ralphs JR (1996) Tendons in health and disease. Man Ther 1:186–191 [DOI] [PubMed]
- 7.Brent AE, Braun T, Tabin CJ (2005) Genetic analysis of interactions between the somitic muscle, cartilage and tendon cell lineages during mouse development. Development 132:515–528 [DOI] [PubMed]
- 8.Brent AE, Schweitzer R, Tabin CJ (2003) A somitic compartment of tendon progenitors. Cell 113:235–248 [DOI] [PubMed]
- 9.Caplan AI (2005) Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng 11:1198–1211 [DOI] [PubMed]
- 10.Chhabra A, Tsou D, Clark RT et al (2003) GDF-5 deficiency in mice delays Achilles tendon healing. J Orthop Res 21:826–835 [DOI] [PubMed]
- 11.Cooper JA Jr, Bailey LO, Carter JN et al (2006) Evaluation of the anterior cruciate ligament, medial collateral ligament, achilles tendon and patellar tendon as cell sources for tissue-engineered ligament. Biomaterials 27:2747–2754 [DOI] [PubMed]
- 12.Forslund C, Aspenberg P (2003) Improved healing of transected rabbit Achilles tendon after a single injection of cartilage-derived morphogenetic protein-2. Am J Sports Med 31:555–559 [DOI] [PubMed]
- 13.Forslund C, Rueger D, Aspenberg P (2003) A comparative dose–response study of cartilage-derived morphogenetic protein (CDMP)-1, -2 and -3 for tendon healing in rats. J Orthop Res 21:617–621 [DOI] [PubMed]
- 14.Garvin J, Qi J, Maloney M, Banes AJ (2003) Novel system for engineering bioartificial tendons and application of mechanical load. Tissue Eng 9:967–979 [DOI] [PubMed]
- 15.Gerich TG, Kang R, Fu FH et al (1997) Gene transfer to the patellar tendon. Knee Surg Sports Traumatol Arthrosc 5:118–123 [DOI] [PubMed]
- 16.Goh JC, Ouyang HW, Teoh SH et al (2003) Tissue-engineering approach to the repair and regeneration of tendons and ligaments. Tissue Eng 9(Suppl 1):S31–S44 [DOI] [PubMed]
- 17.Hildebrand KA, Deie M, Allen CR et al (1999) Early expression of marker genes in the rabbit medial collateral and anterior cruciate ligaments: the use of different viral vectors and the effects of injury. J Orthop Res 17:37–42 [DOI] [PubMed]
- 18.Hildebrand KA, Woo SL, Smith DW et al (1998) The effects of platelet-derived growth factor-BB on healing of the rabbit medial collateral ligament. An in vivo study. Am J Sports Med 26:549–554 [DOI] [PubMed]
- 19.Hoffmann A, Gross G (2006) Tendon and ligament engineering: from cell biology to in vivo application. Regen Med 1:563–574 [DOI] [PubMed]
- 20.Hoffmann A, Pelled G, Turgeman G et al (2006) Neotendon formation induced by manipulation of the Smad8 signalling pathway in mesenchymal stem cells. J Clin Invest 116:940–952 [DOI] [PMC free article] [PubMed]
- 21.Holmbeck K, Bianco P, Caterina J et al (1999) MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell 99:81–92 [DOI] [PubMed]
- 22.Jozsa LG, Kannus P (1997) Human tendons: anatomy, physiology, and pathology. Human Kinetics, Champaign, Illinois
- 23.Juncosa-Melvin N, Boivin GP, Galloway MT et al (2005) Effects of cell-to-collagen ratio in mesenchymal stem cell-seeded implants on tendon repair biomechanics and histology. Tissue Eng 11:448–457 [DOI] [PubMed]
- 24.Kjaer M (2004) Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 84:649–698 [DOI] [PubMed]
- 25.Kjaer M, Langberg H, Miller BF et al (2005) Metabolic activity and collagen turnover in human tendon in response to physical activity. J Musculoskelet Neuronal Interact 5:41–52 [PubMed]
- 26.Lou J, Manske PR, Aoki M, Joyce ME (1996) Adenovirus-mediated gene transfer into tendon and tendon sheath. J Orthop Res 14:513–517 [DOI] [PubMed]
- 27.Lou J, Tu Y, Burns M et al (2001) BMP-12 gene transfer augmentation of lacerated tendon repair. J Orthop Res 19:1199–1202 [DOI] [PubMed]
- 28.Martin RB, Burr DB, Sharkey NA (1998) Skeletal tissue mechanics. Springer, New York
- 29.Mikic B, Schalet BJ, Clark RT et al (2001) GDF-5 deficiency in mice alters the ultrastructure, mechanical properties and composition of the Achilles tendon. J Orthop Res 19:365–371 [DOI] [PubMed]
- 30.Minamitani T, Ikuta T, Saito Y et al (2004) Modulation of collagen fibrillogenesis by tenascin-X and type VI collagen. Exp Cell Res 298:305–315 [DOI] [PubMed]
- 31.Nakamura N, Hart DA, Boorman RS et al (2000) Decorin antisense gene therapy improves functional healing of early rabbit ligament scar with enhanced collagen fibrillogenesis in vivo. J Orthop Res 18:517–523 [DOI] [PubMed]
- 32.Norris RA, Damon B, Mironov V et al (2007) Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem 101:695–711 [DOI] [PMC free article] [PubMed]
- 33.Oliver G, Wehr R, Jenkins NA et al (1995) Homeobox genes and connective tissue patterning. Development 121:693–705 [DOI] [PubMed]
- 34.Page-McCaw A, Ewald AJ, Werb Z (2007) Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 8:221–233 [DOI] [PMC free article] [PubMed]
- 35.Rees JD, Wilson AM, Wolman RL (2006) Current concepts in the management of tendon disorders. Rheumatology (Oxford) 45:508–521 [DOI] [PubMed]
- 36.Schmidt CC, Georgescu HI, Kwoh CK et al (1995) Effect of growth factors on the proliferation of fibroblasts from the medial collateral and anterior cruciate ligaments. J Orthop Res 13:184–190 [DOI] [PubMed]
- 37.Schweitzer R, Chyung JH, Murtaugh LC et al (2001) Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 128:3855–3866 [DOI] [PubMed]
- 38.Settle SH Jr, Rountree RB, Sinha A et al (2003) Multiple joint and skeletal patterning defects caused by single and double mutations in the mouse Gdf6 and Gdf5 genes. Dev Biol 254:116–130 [DOI] [PubMed]
- 39.Sharma P, Maffulli N (2005) Basic biology of tendon injury and healing. Surgeon 3:309–316 [DOI] [PubMed]
- 40.Sharma P, Maffulli N (2006) Biology of tendon injury: healing, modeling and remodeling. J Musculoskelet Neuronal Interact 6:181–190 [PubMed]
- 41.Storm EE, Huynh TV, Copeland NG et al (1994) Limb alterations in brachypodism mice due to mutations in a new member of the TGFb-superfamily. Nature 368:639–643 [DOI] [PubMed]
- 42.Virchenko O, Fahlgren A, Skoglund B, Aspenberg P (2005) CDMP-2 injection improves early tendon healing in a rabbit model for surgical repair. Scand J Med Sci Sports 15:260–264 [DOI] [PubMed]
- 43.Wang Z, Juttermann R, Soloway PD (2000) TIMP-2 is required for efficient activation of proMMP-2 in vivo. J Biol Chem 275:26411–26415 [DOI] [PMC free article] [PubMed]
- 44.Wolfman NM, Hattersley G, Cox K et al (1997) Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest 100:321–330 [DOI] [PMC free article] [PubMed]
- 45.Xu PX, Cheng J, Epstein JA, Maas RL (1997) Mouse Eya genes are expressed during limb tendon development and encode a transcriptional activation function. Proc Natl Acad Sci USA 94:11974–11979 [DOI] [PMC free article] [PubMed]
- 46.Young RG, Butler DL, Weber W et al (1998) Use of mesenchymal stem cells in a collagen matrix for Achilles tendon repair. J Orthop Res 16:406–413 [DOI] [PubMed]
- 47.Zhang G, Ezura Y, Chervoneva I et al (2006) Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem 98:1436–1449 [DOI] [PubMed]


