Abstract
Rapid advancements in the field of genomics, enabled by the achievements of the Human Genome Project and the complete decoding of the human genome, have opened an unimaginable set of opportunities for scientists to further unveil delicate mechanisms underlying the functional homeostasis of biological systems. The trend of applying whole-genome analysis techniques has also contributed to a better understanding of physiological and pathological processes involved in homeostasis of bone and cartilage tissues. Gene expression profiling studies have yielded novel insights into the complex interplay of osteoblast and osteoclast regulation, as well as paracrine and endocrine control of bone and cartilage remodelling. Mechanisms of new bone formation responsible for fracture healing and distraction osteogenesis, as well as healing of joint cartilage defects, have also been extensively studied. Microarray experiments have been especially useful in studying pathological processes involved in diseases such as osteoporosis or bone tumours. Existing results show that microarrays hold great promise in areas such as identification of targets for novel therapies or development of new biomarkers and classifiers in skeletal diseases.
Keywords: Microarrays, Bone biology, Cartilage, Expression profiling, CGH, ChIP-ON-CHIP
Résumé
Les progrès rapides réalisés dans le cadre de la génétique nous ont permis d’achever le projet de génome humain et de compléter son décodage, ceci nous a permis de mieux comprendre également la physiologie et la pathologie de l’homéostasie des tissus osseux cartilagineux, l’expression des gênes interférant sur la régulation des ostéoclastes ou du remodelage osseux par l’intermédiaire d’un contrôle paracrine et endocrine. De même, en ce qui concerne les mécanismes responsables de la consolidation des fractures, de l’ostéogénèse en distraction, de la cicatrisation des lésions cartilagineuses. Ces classifications et ces expérimentations sont également utiles pour comprendre les processus pathologiques tel que l’ostéoporose ou les tumeurs osseuses. Ceci permettra de mettre en route de nouvelles thérapeutiques ou de développer de nouveaux marqueurs afin de pouvoir classer les lésions osseuses.
Introduction
The skeletal system is an extremely dynamic environment which is subjected to constant remodelling. This is achieved through balance of two tightly regulated and complex processes, namely bone formation by osteoblasts and bone resorption by osteoclasts. These two processes are controlled by numerous paracrine and endocrine factors, including osteoblast- and osteoclast-specific signalling proteins and transcription factors, as well as hormones and growth factors. Identification of intrinsic and extrinsic factors responsible for maintenance of bone homeostasis has been the focus of extensive research over the decades, but the complex nature of all the processes involved has severely hampered a more complete understanding. The advent of high-throughput genomic methods, such as microarrays, has finally enabled a comprehensive insight into regulation of bone formation and resorption.
Gene expression profiling studies have mostly concentrated on several major issues, namely osteoblast and osteoclast regulation, endocrine control of the skeletal system, new bone formation in fracture healing and elucidation of pathological processes in diseases such as osteoporosis and bone tumours. Besides these issues, one of the greatest clinical problems is that of damaged or diseased articular cartilage. Therefore, it is no wonder that intensive research of the biological mechanisms of articular cartilage regeneration is being conducted. This wide research also includes the application of gene therapy to damaged cartilage [16, 24]. Prior to specific gene transfer it is necessary to identify genes and proteins responsible for diseased articular cartilage. With this in mind, novel techniques that use microarray methodology in genome1 research and mass spectrometry in proteome2 research are being employed in search of candidate genes and proteins.
Regulation of skeletal homeostasis
Osteoblast and osteoclast differentiation
Osteoblasts and osteoclast differentiation and maintenance have been a major focus of microarray experiments in the last decade. Osteoblasts, which are characterised by their ability to produce and mineralise the bone matrix, differentiate from their mesenchymal precursors in a complex process orchestrated by timely activation of specific transcription factors and hormones. Several transcriptional factors, such as Runx2/Cbfa1 and Osterix [15, 20] have been identified as master regulators of osteoblastic differentiation, whose absence leads to complete lack of mineralised skeleton. Many other transcription factors have also been implied as important regulators of osteoblast differentiation and maintenance such as homeobox proteins, members of the AP1 family or effectors of the β-catenin/Wnt signalling pathway [31]. Microarray studies have shed new light not only on the interactions of well-characterised factors of osteoblast regulation, but have also helped identify novel genes involved in regulation of bone formation. Early studies of osteoblast differentiation performed on MC3T3-E1 cells [1] revealed several distinct functional groups of genes as differentially expressed. These included among others transcription factors [nuclear factor (NF)-κB binding subunit, Sp4 zinc finger, SP2], growth factors [bone morphogenetic protein receptor 1A, fibroblast growth factor (FGF), transforming growth factor (TGF)-β receptor], hormone receptors (oestrogen receptor, calcitonin receptor, insulin receptor) and cell cycle regulators (cyclin A, Cdk4, Cdc 25). Although promising, these early studies were mostly descriptive and represented a useful baseline for more analytical probes of functional pathways. Later studies succeeded in identifying not only well-known regulators, but also novel genes involved in osteoblast differentiation. Using an in vitro model based on mesenchymal progenitor cells treated with dexamethasone, ascorbic acid and β-glycerol phosphate, Qi et al. [26] found several differentially expressed genes, not previously known to be involved in osteoblast differentiation, which co-clustered in expression profile with osteoblast-specific transcription factors. These included many members of the zinc finger family of proteins (zinc finger 177, 143, 133) which were significantly upregulated. Differentially upregulated genes also included SOX-4, SOX-22 genes, MAD4 and CRABP1. Novel downregulated transcripts included MYC, transcription factor CA150 and transcription elongation factor B.
In a more recent study, Kalajzic et al. [12] used collagen 1a1 promoter-green fluorescent protein (GFP) transgenic mouse lines to isolate bone cells at distinct stages of osteoprogenitor maturation. This was done in an effort to overcome the inherent heterogeneity of bone cells. Well-characterised functional groups of genes involved in osteoblast differentiation were significantly enriched in GFP sorted cells and included genes from the insulin-like growth factor (IGF) 1 and 2 pathways, members of the bone morphogenetic protein family (BMP8a, BMP2, Smad1, Smad5) and genes pertaining to the Wnt pathway (Wnt3a, Wnt5a, Frizzled1).
Gene expression studies of osteoclastogenesis have also provided extensive insight into the balance of bone homeostasis. Cytokines, such as macrophage colony-stimulating factor (M-CSF) and receptor activator of NF-κB ligand (RANKL), have been shown to induce differentiation of bone marrow haematopoietic precursor cells into bone-resorbing osteoclasts without requiring stromal cells of mesenchymal origin [2]. Using an autonomous mouse osteoclastogenesis model consisting of bone marrow cells cultured with soluble forms of M-CSF and RANKL, Cappellen et al. [2] showed that M-CSF alone introduced not only RANKL but also other components of the RANK/NF-κB pathway, such as TRAF2A, MEKK3 and RIPK1. M-CSF also lead to induction of interleukins, interferons and their receptors (IL-1a, IL-18, IL-6, IFN-β). RANKL alone induced a specific set of 70 novel genes, including chemokines and growth factors [RANTES, platelet-derived growth factor (PDGF)α, IGF-1]. The study concludes that M-CSF mainly induced expression of genes necessary for a direct response to RANKL and interleukin, while RANKL directed a three-stage differentiation programme and induced genes for interaction with osteoblasts and immune and nerve cells. These results indicate collectively that an important interplay exists between osteoclasts, osteoblasts and other cells involved in bone remodelling.
This was further substantiated in two other studies of osteoclastogenesis. Ishida et al. [11] analysed gene expression profiles of mouse RAW264 cells treated with RANKL. Among the 106 early inducible genes, the authors identified four genes, namely syndecan, neoplastic progression 3, DSCR1 and NFAT2, as being overexpressed and correlated with induction of matured osteoclasts. Of these NFAT2 was shown to play a key role in osteoclastogenesis. Subsequent study showed inhibition of bone formation in NFAT1- and NFAT2-deficient cells [14]. Further investigation into the mechanisms of bone formation showed that NFAT1 stimulates Osterix-dependent activation of the Col1a1 promoter, but not Runx2-dependent activation of Bglap1 (osteocalcin) promoter. In addition, NFAT and Osterix form a complex that binds to DNA, which is necessary for transcriptional activity of Osterix and points to co-operative regulation of osteoclastogenesis and osteoblastic bone formation.
Recently a huge amount of interest has been directed towards the Wnt signalling pathway in bone remodelling [7]. Wnt signalling proteins are highly conserved secreted molecules that regulate embryonic cell fates by enabling embryonic decisions. Canonical Wnt signalling has been recognised as a key regulator of the osteoblastogenesis, chondrogenesis and formation of the skeleton in embryonic development. In response to Wnt signalling β-catenin, the main signalling molecule, is stabilised, accumulates in the cytoplasm, enters the nucleus, where partnered with LEF/TCF transcription factors activates new gene expression programmes, among others, c-myc and cyclin D1. When the signal is absent, β-catenin is being ubiquinated and destroyed by the proteasome. The inhibitor of the Wnt signalling Dickkopf-1 (DKK-1) is the main regulator of joint remodelling [6]. Low levels of DKK-1 relieved Wnt signalling and enabled the formation of osteophytes, while increased levels of DKK-1 found in rheumatoid arthritis patients inhibited Wnt signalling and impaired bone formation. Seemingly, the Wnt pathway is controlling the mechanisms of both bone formation and resorption in human joint disease (Fig. 1).
Fig. 1.
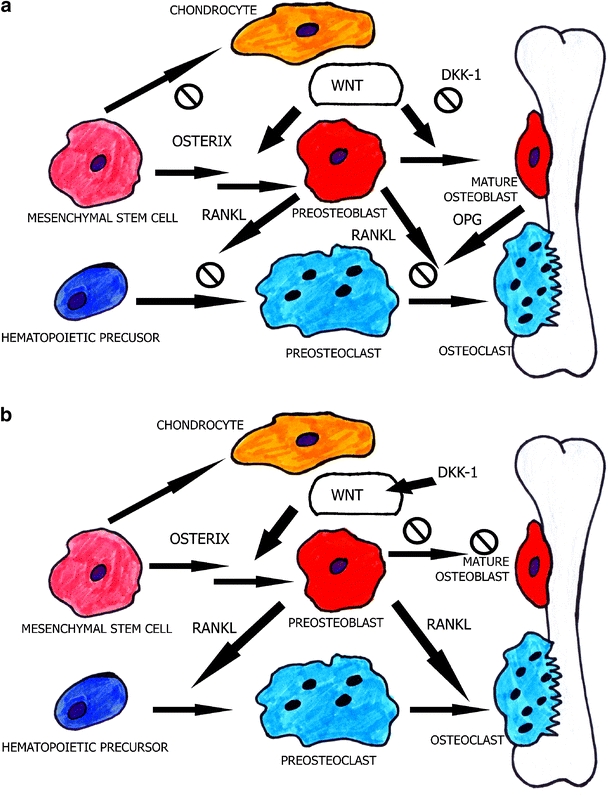
Mechanisms of osteoblast and osteoclast function induced by Wnt signalling. a When the pathway is activated, osteoblasts differentiate and through mature osteoblast upregulation of OPG inhibit RANKL-induced osteoclastogenesis preventing bone resorption. b When the pathway is arrested, osteoblast proliferation and differentiation also cease, causing the precursors of mature osteoblasts to activate RANKL. Osteoclastogenesis and bone resorption follow. OPG osteoprotegerin, RANKL receptor activator of NF-κB ligand (modified from [8])
Endocrine regulation of bone remodelling
Systemic effects of hormones and growth factors play an important role in physiological and pathological mechanisms of bone remodelling. Oestrogen and parathyroid hormone (PTH) have been among the most intensively studied, because of the fact that both are currently used in the therapy of osteoporosis. Studies on the effect of oestrogen in ovariectomised (OVX) mice [17] revealed several genes already known to be regulated by oestrogen, but also new possible targets, such as IL-1 receptor antagonist, IL-1 receptor type II, insulin-like growth factor-binding protein 4 and transforming growth factor β. Gene expression profiling experiments of ROS17/2.8 cells treated with 17β-oestradiol (E2) showed that p53 plays a pivotal role in osteoblast response to oestrogen treatment [4]. P53 exhibited a biphasic change in transcription activation, with specific induction of apoptosis- and cell cycle arrest-related genes, as well as survival pathway genes. In conclusion, the authors show that p53 plays an important role in osteoblast differentiation and interaction of E2 and p53 might hold a vital position in osteoblast maturation.
Although primarily known as a major bone resorption hormone, PTH has been recently approved as a first bone anabolic therapy for treatment of osteoporosis. In bone, sustained release of PTH in serum leads to release of calcium from bones, mainly through degradation of bone matrix by osteoclasts. This is known to be an indirect effect which requires prior PTH activation of osteoblasts. Despite the fact that net stimulation of bone resorption occurs during sustained PTH excess, intermittent administration of PTH leads to overall anabolic effect and large increases in bone mineral density (BMD). To further study effects of sustained increased levels of PTH on bone remodelling, Reppe et al. [27] analysed frozen bone biopsies of patients with primary hyperparathyroidism using microarrays. Most of the differentially expressed genes represented structural and adhesion proteins, but included also proteases and protease regulators indicating increased bone resorption. Expression of collagen type I and osteocalcin was also significantly increased pointing to the aforementioned PTH anabolic action.
Fracture healing and distraction osteogenesis
The possible mechanism of enhanced fracture healing has been one of the major motives for expression profiling studies of bone repair. In order to study differential expression at various stages of fracture healing, Rundle et al. [29] studied in rats expression of genes at day 3 following fracture, immediately after the inflammatory phase but prior to bone formation, and at day 11, when intramembranous and enchondral bone formation overlap. Several Gene Ontology categories were represented that suggested important regulatory pathways active at specific time points. The cell proliferation and protein metabolism categories were well represented at day 3, indicating proliferation of periosteal mesenchymal cells of the early soft callus. Several members of the skeletal development, cell adhesion and extracellular matrix categories were present at day 11 of healing, consistent with the maturation of the various callus tissues during enchondral bone formation. Among individual genes, PDGF was significantly expressed at day 3, while TGF-β, vascular endothelial growth factor (VEGF)-C and hepatocyte growth factor exhibited increased expression at day 11 following fracture.
Distraction osteogenesis represents a unique and effective way to treat many congenital and post-traumatic musculoskeletal problems. Studies of biological mechanisms involved in distraction osteogenesis demonstrated that angiogenesis contributes significantly to fracture healing during distraction osteogenesis. Results of a study conducted on a rat model [23] revealed increased mRNA expression for a wide variety of angiogenic factors including angiopoietin (Ang) 1 and 2, Tie 1 and 2 receptors, VEGF-A and -D, VEGF receptor 2 and neuropilin 1. Expression of these factors was found to be maximal during the phase of active distraction. A similar study conducted on mice [3] showed once again increased expression of Ang 1 and 2, Tie 2 receptor and VEGF-A. In addition, a key transcriptional regulator of angiogenic factors, hypoxia-induced factor-1α, was also overexpressed, as well as FGF-binding protein and multiple matrix metalloproteinases. These data suggest that bone formation during distraction osteogenesis is accompanied by the robust induction of factors associated with angiogenesis.
Pathophysiological processes in bone biology
Diseases such as osteoporosis and bone tumours represent a significant health-related problem in the twenty-first century. The well-accepted pathophysiological mechanisms for low bone mass include prolongation of the life span of osteoclasts and early apoptosis of osteoblasts and osteocytes. Several studies have used both genetic and genomic approaches in order to further clarify the mechanisms which regulate this apparent discrepancy in the life span of osteoclasts and osteoblasts. A study by Klein et al. [13] identified the lipoxygenase gene Alox15 as a major negative regulator of peak bone mineral density in mice. This was confirmed both in Alox15 knockout mice and in studies using pharmacological inhibitors of the enzyme. A more recent study [18] used the approach of expression profiling of circulating monocytes, possible progenitors of osteoclasts, isolated from patients with low BMD. The results of the study confirmed a strong correlation between upregulation of chemokine receptor 3, histidine decarboxylase and glucocorticoid receptor with low BMD Z-scores in patients. Our group has recently published a study [22] aimed at identifying not only differentially expressed genes, but also significantly enriched pathways in bones of OVX rats. Among bone-specific genes we observed upregulation of interleukin 7 (IL-7), IL-7 receptor and matrix metallopeptidase 8, while genes such as transforming growth factor β-3, procollagen type I and procollagen type VI exhibited a marked decrease in expression. Using gene set enrichment analysis we tried to identify functional sets specifically enriched in OVX animals. The control animals exhibited marked enrichment of insulin-like growth factor 1 (IGF-1)-related pathways, including IGF-1 receptor pathway, insulin signalling pathway, glycogen metabolism, as well as glycolysis and gluconeogenesis. On the other hand, OVX animals showed increased enrichment of fatty acid metabolism, IL-12 and tumour necrosis factor receptor 2 (TNFR2) pathways, indicating increased cytokine production in OVX animals. Enrichment of the caspase pathway might indicate an increased rate of apoptosis in these animals. Furthermore, the proteasome pathway was also significantly enriched following ovariectomy, pointing to increased protein degradation.
Several tumours of the skeletal system have so far been thoroughly analysed. Using a novel approach of array-based comparative genomic hybridisation, Heidenblad et al. [10] tried to identify DNA copy number alterations using 6 bone and 15 deep-seated tissue tumour samples from 19 patients. More than 40% of all alterations were mapped to chromosome 12, and 8 of 11 cases with the 12q amplification showed involvement of the c-jun NH2-terminal kinase/mitogen-activated protein kinase pathway, indicating that aberrant expression of genes involved may represent an important step in tumour dedifferentiation. A study aimed at clarifying the mechanisms of malignant progression from benign enchondromas to chondrosarcomas indicated a possible role in alteration of DNA copy number in genes encoding ribosomal protein S6 and cyclin-dependent kinase 4 [28]. Expression profiling studies have, on the other hand, indicated the decrease in Indian hedgehog signalling as a major component of chondrosarcoma progression [9].
Pathophysiological processes in cartilage biology
Extensive efforts have been made in searching for the repair mechanisms of articular cartilage. Since no natural repair mechanisms have been identified so far, one of the obvious approaches to addressing this very important clinical problem is the use of gene transfer in gene therapy approaches. Therefore, microarray techniques are irreplaceable methods for prior identification of relevant genes involved in damaged cartilage. So far some of the cDNAs have been found to be useful in repairing the damaged cartilage [25]. These include members of the bone morphogenetic proteins (BMPs) family, several TGF-β superfamily members, growth factors IGF-1, FGF and epidermal growth factor (EGF), as well as transcription factors Sox-9, Sox-6 and L-Sox-5. Some of the signal transduction molecules such as SMADS are also involved.
Future directions
The majority of previous microarray studies have been focused mainly on elucidating the underlying biological mechanisms of bone biology. With the fruition of microarray technology application-driven studies have become more common, especially in the field of bone tumour biology. One such example is a study by Ohali et al. [21] in which the authors tried to develop a genomic classifier able to distinguish between poor and good prognosis patients with Ewing’s sarcoma (ES). The results of the study led to identification of two distinct gene expression signatures distinguishing high-risk ES patients prone to disease progression from low-risk ES patients with a favourable prognosis for long-term progression-free survival. The microarray-based classification was superior to currently used prognostic parameters. The chromatin immunoprecipitation approach was used in analysis of EWS-FL11-specific promoter targets in a cell model of ES [30]. The results were extremely promising, identifying MK-STYX, which encodes a MAP kinase phosphatase-like protein, as a possible target for future drug development.
Mintz et al. [19] used expression profiling approach in order to identify an expression signature able to classify chemotherapy-resistant paediatric osteosarcomas. They were able to identify several genes involved in tumour progression, ECM remodelling and osteoclastogenesis which may contribute to chemotherapy-resistant phenotype. In a recent study Dalla-Tore et al. [5] used a pathway-focused approach by analysing osteosarcoma with low density expression cDNA arrays to screen for candidate genes related to tumour progression. Among the differentially expressed genes, THB53 and SPARC were identified as markers of poor response to chemotherapy and worse overall survival. On the other hand, SPP1 was found to be a marker of better overall survival.
The future of genomic approaches in orthopaedic surgery holds within itself great promises, which will lead to development of novel diagnostic and prognostic tests and procedures. Furthermore, application of ever-developing genomic technologies may lead to discovery of novel drug therapies as well as design of individually tailored therapeutic paradigms, ushering in a new era of molecular medicine.
Footnotes
The genome is the complete set of sequences in the genetic material of an organism. It includes the sequences of each chromosome plus any DNA in organelles.
The proteome is the complete set of proteins that is expressed by the entire genome. Because some genes code for multiple proteins, the size of the proteome is greater than the number of genes.
References
- 1.Beck GR Jr, Zerler B, Moran E (2001) Gene array analysis of osteoblast differentiation. Cell Growth Differ 12:61–83 [PubMed]
- 2.Cappellen D, Luong-Nguyen NH, Bongiovanni S, Grenet O, Wanke C, Susa M (2002) Transcriptional program of mouse osteoclast differentiation governed by the macrophage colony-stimulating factor and the ligand for the receptor activator of NFkappa B. J Biol Chem 277:21971–21982 [DOI] [PubMed]
- 3.Carvalho RS, Einhorn TA, Lehmann W, Edgar C, Al-Yamani A, Apazidis A, Pacicca D, Clemens TL, Gerstenfeld LC (2004) The role of angiogenesis in a murine tibial model of distraction osteogenesis. Bone 34:849–861 [DOI] [PubMed]
- 4.Chandar N, Logan D, Szajkovics A, Harmston W (2004) Gene expression changes accompanying p53 activity during estrogen treatment of osteoblasts. Life Sci 75:2045–2055 [DOI] [PubMed]
- 5.Dalla-Torre CA, Yoshimoto M, Lee CH, Joshua AM, de Toledo SR, Petrilli AS, Andrade JA, Chilton-MacNeill S, Zielenska M, Squire JA (2006) Effects of THBS3, SPARC and SPP1 expression on biological behavior and survival in patients with osteosarcoma. BMC Cancer 6:237 [DOI] [PMC free article] [PubMed]
- 6.Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, Korb A, Smolen J, Hoffmann M, Scheinecker C, van der Heide D, Landewe R, Lacey D, Richards WG, Schett G (2007) Dickkopf-1 is a master regulator of joint remodeling. Nat Med 13:156–163 [DOI] [PubMed]
- 7.Glass DA, Karsently G (2007) In vivo analysis of Wnt signaling in bone. Endocrinology. DOI 10.1210/en.2006-1372 [DOI] [PubMed]
- 8.Goldring SR, Goldring MB (2007) Eating bone or adding it: the Wnt pathway decides. Nat Med 13:133–134 [DOI] [PubMed]
- 9.Hameetman L, Rozeman LB, Lombaerts M, Oosting J, Taminiau AH, Cleton-Jansen AM, Bovee JV, Hogendoorn PC (2006) Peripheral chondrosarcoma progression is accompanied by decreased Indian Hedgehog signalling. J Pathol 209:501–511 [DOI] [PubMed]
- 10.Heidenblad M, Hallor KH, Staaf J, Jonsson G, Borg A, Hoglund M, Mertens F, Mandahl N (2006) Genomic profiling of bone and soft tissue tumors with supernumerary ring chromosomes using tiling resolution bacterial artificial chromosome microarrays. Oncogene 25:7106–7116 [DOI] [PubMed]
- 11.Ishida N, Hayashi K, Hoshijima M, Ogawa T, Koga S, Miyatake Y, Kumegawa M, Kimura T, Takeya T (2002) Large scale gene expression analysis of osteoclastogenesis in vitro and elucidation of NFAT2 as a key regulator. J Biol Chem 277:41147–41156 [DOI] [PubMed]
- 12.Kalajzic I, Staal A, Yang WP, Wu Y, Johnson SE, Feyen JH, Krueger W, Maye P, Yu F, Zhao Y, Kuo L, Gupta RR, Achenie LE, Wang HW, Shin DG, Rowe DW (2005) Expression profile of osteoblast lineage at defined stages of differentiation. J Biol Chem 280:24618–24626 [DOI] [PubMed]
- 13.Klein RF, Allard J, Avnur Z, Nikolcheva T, Rotstein D, Carlos AS, Shea M, Waters RV, Belknap JK, Peltz G, Orwoll ES (2004) Regulation of bone mass in mice by the lipoxygenase gene Alox15. Science 303:229–232 [DOI] [PubMed]
- 14.Koga T, Matsui Y, Asagiri M, Kodama T, de Crombrugghe B, Nakashima K, Takayanagi H (2005) NFAT and Osterix cooperatively regulate bone formation. Nat Med 11:880–885 [DOI] [PubMed]
- 15.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T (1997) Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 30:755–764 [DOI] [PubMed]
- 16.Lind M, Bunger C (2005) Orthopaedic applications of gene therapy. Int Orthop 29:205–209 [DOI] [PMC free article] [PubMed]
- 17.Lindberg MK, Moverare S, Eriksson AL, Skrtic S, Gao H, Dahlman-Wright K, Gustafsson JA, Ohlsson C (2002) Identification of estrogen-regulated genes of potential importance for the regulation of trabecular bone mineral density. J Bone Miner Res 17:2183–2195 [DOI] [PubMed]
- 18.Liu YZ, Dvornyk V, Lu Y, Shen H, Lappe JM, Recker RR, Deng HW (2005) A novel pathophysiological mechanism for osteoporosis suggested by an in vivo gene expression study of circulating monocytes. J Biol Chem 280:29011–29016 [DOI] [PubMed]
- 19.Mintz MB, Sowers R, Brown KM, Hilmer SC, Mazza B, Huvos AG, Meyers PA, Lafleur B, McDonough WS, Henry MM, Ramsey KE, Antonescu CR, Chen W, Healey JH, Daluski A, Berens ME, Macdonald TJ, Gorlick R, Stephan DA (2005) An expression signature classifies chemotherapy-resistant pediatric osteosarcoma. Cancer Res 65:1748–1754 [DOI] [PubMed]
- 20.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B (2002) The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108:17–29 [DOI] [PubMed]
- 21.Ohali A, Avigad S, Zaizov R, Ophir R, Horn-Saban S, Cohen IJ, Meller I, Kollender Y, Issakov J, Yaniv I (2004) Prediction of high risk Ewing’s sarcoma by gene expression profiling. Oncogene 23:8997–9006 [DOI] [PubMed]
- 22.Orlic I, Borovecki F, Simic P, Vukicevic S (2007) Gene expression profiling in bone tissue of osteoporotic mice. Arh Hig Rada Toksikol 58:3–11 [DOI] [PubMed]
- 23.Pacicca DM, Patel N, Lee C, Salisbury K, Lehmann W, Carvalho R, Gerstenfeld LC, Einhorn TA (2003) Expression of angiogenic factors during distraction osteogenesis. Bone 33:889–898 [DOI] [PubMed]
- 24.Pecina M, Jelic M, Ivkovic A, Hudetz D (2006) Gene therapy applications in orthopaedics. Int Orthop 30:215–216 [DOI] [PMC free article] [PubMed]
- 25.Pecina M, Jelic M, Martinovic S, Haspl M, Vukicevic S (2002) Articular cartilage repair: the role of bone morphogenetic proteins. Int Orthop 26:131–136 [DOI] [PMC free article] [PubMed]
- 26.Qi H, Aguiar DJ, Williams SM, La Pean A, Pan W, Verfaillie CM (2003) Identification of genes responsible for osteoblast differentiation from human mesodermal progenitor cells. Proc Natl Acad Sci U S A 100:3305–3310 [DOI] [PMC free article] [PubMed]
- 27.Reppe S, Stilgren L, Olstad OK, Brixen K, Nissen-Meyer LS, Gautvik KM, Abrahamsen B (2006) Gene expression profiles give insight into the molecular pathology of bone in primary hyperparathyroidism. Bone 39:189–198 [DOI] [PubMed]
- 28.Rozeman LB, Szuhai K, Schrage YM, Rosenberg C, Tanke HJ, Taminiau AH, Cleton-Jansen AM, Bovee JV, Hogendoorn PC (2006) Array-comparative genomic hybridization of central chondrosarcoma: identification of ribosomal protein S6 and cyclin-dependent kinase 4 as candidate target genes for genomic aberrations. Cancer 107:380–388 [DOI] [PubMed]
- 29.Rundle CH, Wang H, Yu H, Chadwick RB, Davis EI, Wergedal JE, Lau KH, Mohan S, Ryaby JT, Baylink DJ (2006) Microarray analysis of gene expression during the inflammation and endochondral bone formation stages of rat femur fracture repair. Bone 38:521–529 [DOI] [PubMed]
- 30.Siligan C, Ban J, Bachmaier R, Spahn L, Kreppel M, Schaefer KL, Poremba C, Aryee DN, Kovar H (2005) EWS-FLI1 target genes recovered from Ewing’s sarcoma chromatin. Oncogene 24:2512–2524 [DOI] [PubMed]
- 31.Stains JP, Civitelli R (2003) Genomic approaches to identifying transcriptional regulators of osteoblast differentiation. Genome Biol 4:222.1–222.4 [DOI] [PMC free article] [PubMed]


