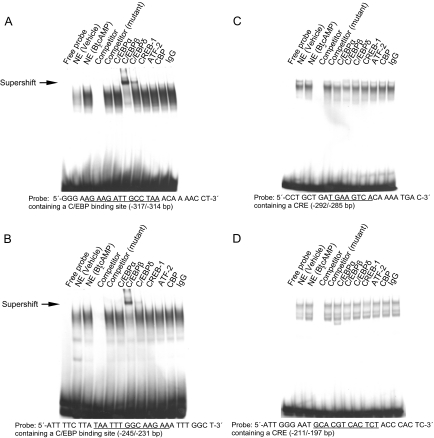
Figure 3.
Characterization of protein binding to the cis-regulatory elements on the aromatase promoter I.3/II region in LSMCs. EMSA was performed using oligonucleotide probes containing a C/EBP or CRE cis-regulatory element from the aromatase promoter I.3/II region, as indicated. All nuclear extracts (except the vehicle lane) were obtained from LSMCs treated with Bt2cAMP (100 μm, 24 h). Unlabeled oligonucleotide was used as cold probe competitor, and competitors of mutated consensus sequences of the C/EBP binding site and CRE were used to confirm specificity of binding activity. Antibodies against C/EBPα, C/EBPβ, C/EBPδ, CREB-1, ATF-2, and CBP were used in supershift assays to identify proteins binding to each cis-regulatory element probe, and nonimmune IgG was used as a negative control. Distinct nuclear protein-DNA complexes occupied two C/EBP binding sites within the −317/−304 bp (A) and −245/−231 bp (B) probes. One of these complexes was supershifted upon addition of anti-C/EBPβ antibody and, although less prominently, anti-C/EBPδ antibody (arrow). Distinct nuclear protein-DNA complexes also occupied two CREs located at −292/−285 bp (C) and −211/−197 bp (D), but these complexes could not be supershifted with addition of any antibody tested. NE, nuclear extract; IgG, nonimmune rabbit IgG.