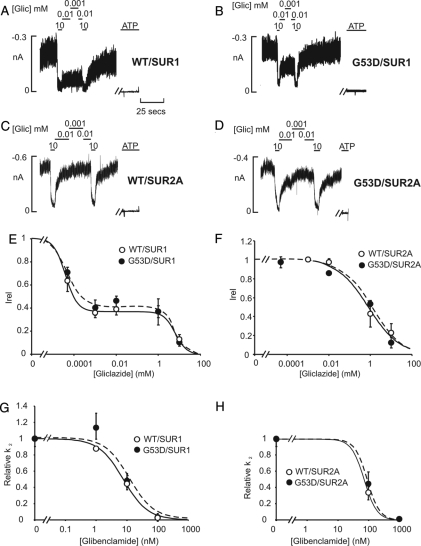
Figure 4.
Unaltered SU sensitivity of KATP currents from COS cells expressing G53D-containing channels. A, Representative currents recorded from inside-out membrane patches from COS cells expressing Kir6.2 WT or G53D, coexpressed with either SUR1 (A and B) or SUR2A (C and D) at −50 mV. Patches were exposed to differing gliclazide, and baseline current was determined by addition of either ATP (5 mm). E and F, Steady-state dependence of membrane current on gliclazide [relative to current in zero tolbutamide (Irel)] for wild-type (WT) and mutant G53D channels. Data points represent the mean ± sem (n = 3–7 patches). The fitted lines correspond to least-square fits of a double-Hill equation (see Patient and Methods). For WT + SUR1 channels: the half-inhibitory concentration of the high-affinity site (Ki1/2, high) = 4.1 μm and Hill coefficient of high-affinity site (HA) = 1.5, and the low-affinity site (Ki1/2, low) = 6.8 mm and Hill coefficient of low-affinity site (HB) = 1.5, the fraction of the high-affinity site (f) = 0.63. For G53D + SUR1 channels: Ki1/2, high = 5 μm, and HA = 1.2, Ki1/2, low = 6.6 mm, and HB = 2, and f = 0.60. For WT + SUR2A channels: Ki1/2, low = 0.8 mm, and H = 0.6. For G53D + SUR2A channels: Ki1/2, low = 1.2 mm, and H = 0.6. G and H, Steady-state dependence of 86Rb+ efflux on glibenclamide for WT and G53D channels coexpressed with either SUR1 (G) or SUR2A (H) in metabolic inhibition (oligomycin and 2-deoxyglucose). Intact cells were exposed to differing glibenclamides, and baseline current was calculated in 1 μm glibenclamide. This concentration of glibenclamide saturates the high-affinity component of block (28). Data points represent the mean ± sem (n = 3–5 experiments). Data were fit with a Hill equation (see Patient and Methods). For WT + SUR1: Ki1/2 = 7.6 nm, and H = 1.2; G53D + SUR1: Ki1/2 = 11.1 nm, and H = 1.1. For WT + SUR2A: Ki1/2 = 73.2 nm, and H = 2.1; G53D + SUR2A: Ki1/2 = 92.3 nm, and H = 2.2.