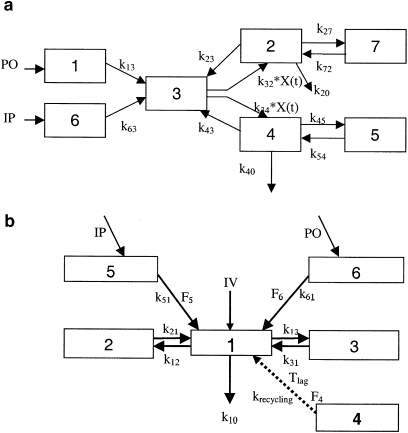
Figure 1.
Pharmacokinetic (PK) models accounting for enterohepatic recycling. (a) PK model for diclofenac and its metabolite 4-hydroxydiclofenac. CMT1 represents the administration site for p.o. dosing, CMT6 represents the administration site for i.p. dosing, CMT2 represents the central compartment for the parent, CMT7 describes the disposition of the parent, CMT4 represents the central compartment for the metabolite, CMT5 describes the disposition of the metabolite and CMT3 represents the enterohepatic recirculation (EHC) compartment. The periodic transfer rate of EHC to central compartment is a nonlinear function (k32*X(t)and k34*X(t)). See PK data analysis for further details. (b) Mixture model with a conversion compartment for rofecoxib. CMT1–CMT3 depict the central and peripheral compartments, respectively. CMT5 and CMT6 represent the depot compartments following i.p. and p.o. administration of rofecoxib. EHC is described by CMT4, Tlag is the lag time associated with the start of EHC and krecycling the re-absorption rate constant. F4–F6 are estimates of the bioavailability for compartments 4–6, respectively. Dashed arrow represents administration of a fictitious dose into the EHC compartment.