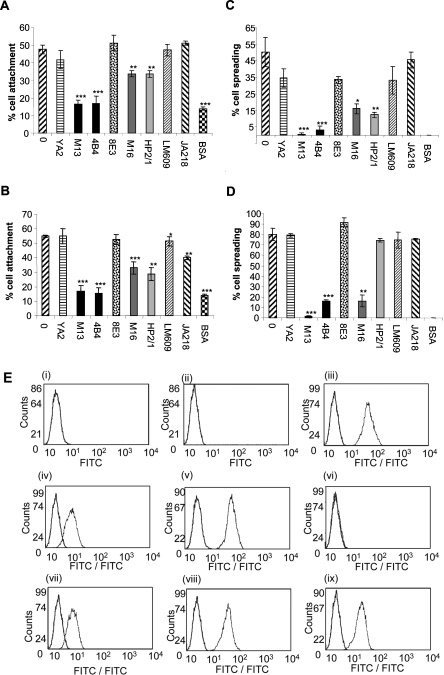
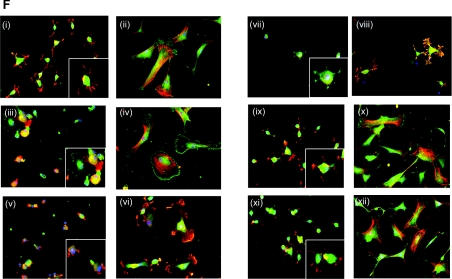
Figure 3. Integrins that mediate SMC attachment and spreading on fibulin-5.
(A–D) Anti-integrin mAb inhibition of SMC attachment (A, B) and spreading (C, D) on full-length fibulin-5 (A, C) or fibronectin (B, D). Y9A2 is an inhibitory α9β1 antibody, mAb13 and 4B4 are both inhibitory anti-β1 antibodies, 8E3 is a non-inhibitory anti-αβ1 antibody, mAb16 is an inhibitory anti-α5 antibody, HP2/1 is an inhibitory α4β1 antibody, LM609 is an inhibitory anti-αVβ3 antibody, and JA218 is an inhibitory anti-α2 antibody. Antibodies were used at 10 μg/ml. BSA indicates background cell attachment to BSA-blocked wells. Results are presented as the means±S.D. for three repeated experiments and were statistically analysed using unpaired Student's t tests (GraphPad Prism 2.0). Results are statistically significant when the P value is <0.05 (*P<0.05, **P<0.001 and ***P<0.0001). SMC adhesion to fibulin-5 was blocked by the β1 function-blocking antibodies, mAb13 and 4B4 (***P<0.0001 for both, each compared with untreated SMCs on fibulin-5). The α5 (mAb16) and α4 (HP2/1) antibodies reduced cell adhesion (**P<0.001 for both, each compared with untreated SMCs on fibulin-5). The LM609 antibody to integrin αvβ3 showed no inhibition (P=0.9282 compared with untreated SMCs on fibulin-5). Antibodies to α9β1 (Y9A2), α2 (JA218) and the non-inhibitory β1 mAb (8E3) all failed to significantly inhibit adhesion (P=0.2730, P=0.4021 and P=0.4215 respectively, each compared with untreated SMCs on fibulin-5). SMC spreading on fibulin-5 was also significantly blocked by antibodies to β1, α5 and α4 integrin subunits (Figure 4C) (***P<0.0001, *P<0.05 and **P<0.001 respectively, each compared with untreated SMCs on fibulin-5). All assays were repeated at least three times, and a representative experiment is shown. (E) FACS detection of SMC cell-surface integrins using the anti-α2 antibody JA218 (iii), anti-α4 antibody HP2/1 (iv), anti-α5 antibody mAb16 (v), anti-α9β1 antibody Y9A2 (vi), anti-αvβ3 antibody LM609 (vii), anti-αvβ5 antibody P1F6 (viii), and anti-β1 antibody 8E3 (ix). Anti-(mouse IgG) antibody (i) and anti-(rat IgG) antibody (ii) were used as negative controls. Antibodies were used at 10 μg/ml. Each integrin detection peak (green) is overlaid with the appropriate IgG negative control (black). (F) Immunofluorescence images of mAb effects on SMC morphology on 250 nM full-length fibulin-5 (i, iii, v, vii, ix and xi) or fibronectin (ii, iv, vi, viii, x and xii) stained for phalloidin (red), and immunostained for the cytoskeletal component, paxillin (green). mAbs to integrin subunits α4 (iii and iv), α5 (v and vi) and β1 (vii and viii), and integrins αvβ3 (ix and x) and αvβ5 (xi and xii) were added (10 μg/ml) at the time of cell seeding. SMCs on full-length fibulin-5 or fibronectin with no antibody (i and ii) were used as negative controls. The anti-β1 mAbs caused cellular rounding and loss of lamellipodia. Images are shown at ×20 (i, iii, v, vii, ix and xi) and ×40 (ii, iv, vi, viii, x and xii; and insets) magnifications for each condition.