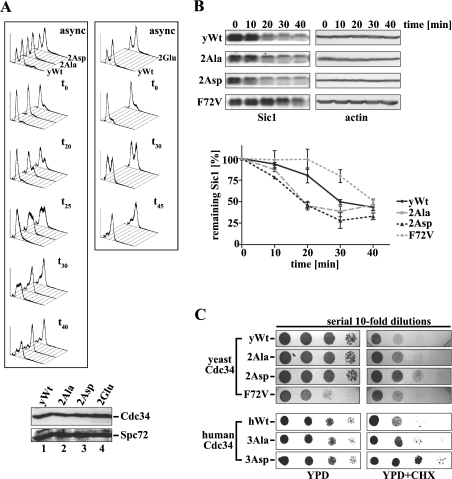
Figure 6. Mutation of the yCdc34 phosphorylation sites affects cell cycle progression and Sic1 turnover in vivo.
(A) Exponentially, asynchronously (async) growing yeast cells (YMS034) expressing wild-type yCdc34 (yWt) or the phosphosite mutants 2Ala, 2Asp (left-hand panel) and 2Glu (right-hand panel) were synchronized in G1-phase by α-factor arrest, released into the cell cycle by incubating in fresh medium, and aliquots were withdrawn at the time points indicated (t0=0 min). Cellular DNA was stained with propidium iodide and analysed by flow cytometry. The histograms are representative of at least three independent experiments. Equivalent expression levels of wild-type (lane 1) and mutant yCdc34s (lanes 2–4) were confirmed by immunoblotting of protein lysates of cells with anti-Cdc34 antibody (upper panel) and even loading was confirmed by immunoblotting with anti-Spc72 antibody (lower panel). (B) Yeast strains expressing either wild-type yCdc34 (yWt), 2Ala, 2Asp or F72V were synchronized in G1 of the cell cycle by α-factor arrest. At the indicated times after release, cell extracts were prepared and analysed by immunoblotting with Sic1 antibody (left-hand panel) and actin antibody as a loading control (right-hand panel). Sic1 levels of cells expressing wild-type yCdc34 (solid black line), F72V (dotted grey line) and the phosphosite mutants 2Ala (dashed grey line) and 2Asp (dotted black line) of three independent experiments were quantified (lower panel). The data points represent the means±S.D. (n=3). (C) Mutation of the phosphorylation sites confers increased resistance on cycloheximide (CHX). Serial 10-fold dilutions of yeast cells (YES71) containing either yeast wild-type Cdc34 (yWt) or the phosphosite mutants 2Ala, 2Asp and 2Glu or the point mutant yCdc34(F72V) were spotted on to YPD plates containing no (upper left panel) or 1.25 μg/ml CHX (upper right panel) and then incubated at 30 °C. Yeast cells containing human wild-type Cdc34 (hWt) or the phosphosite mutants 3Ala and 3Asp were subjected to the same analysis (lower panels) except that 0.75 μg/ml CHX was employed.