Abstract
Bnip3 is a pro-apoptotic member of the Bcl-2 family that is down-regulated in pancreatic cancers, which correlates with resistance to chemotherapy and a worsened prognosis. In contrast, Bnip3 is up-regulated in heart failure and contributes to loss of myocardial cells during I/R (ischaemia/reperfusion). Bnip3 exerts its action at the mitochondria, but the mechanism by which Bnip3 mediates mitochondrial dysfunction is not clear. In the present study, we have identified Bax and Bak as downstream effectors of Bnip3-mediated mitochondrial dysfunction. Bnip3 plays a role in hypoxia-mediated cell death, but MEFs (mouse embryonic fibroblasts) derived from mice deficient in Bax and Bak were completely resistant to hypoxia even with substantial up-regulation of Bnip3. These cells were also resistant to Bnip3 overexpression, but re-expression of Bax or Bak restored susceptibility to Bnip3, suggesting that Bnip3 can act via either Bax or Bak. In contrast, Bnip3 overexpression in wild-type MEFs induced mitochondrial dysfunction with loss of membrane potential and release of cytochrome c. Cell death by Bnip3 was reduced in the presence of mPTP (mitochondrial permeability transition pore) inhibitors, but did not prevent Bnip3-mediated activation of Bax or Bak. Moreover, overexpression of Bnip3ΔTM, a dominant-negative form of Bnip3, reduced translocation of GFP (green fluorescent protein)–Bax to mitochondria during sI/R (simulated I/R) in HL-1 myocytes. Similarly, down-regulation of Bnip3 using RNA interference decreased activation of Bax in response to sI/R in HL-1 myocytes. These results suggest that Bnip3 mediates mitochondrial dysfunction through activation of Bax or Bak which is independent of mPTP opening.
Keywords: apoptosis, Bak, Bax, Bnip3, ischaemia/reperfusion, mitochondrial permeability transition pore
Abbreviations: ΔΨm, mitochondrial membrane potential; BA, bongkrekic acid; BH domain, Bcl-2 homology domain; CsA, cyclosporin A; DKO, double knockout; Drp-1, dynamin-related protein 1; FBS, foetal bovine serum; GFP, green fluorescent protein; I/R, ischaemia/reperfusion; LDH, lactate dehydrogenase; MEF, mouse embryonic fibroblast; mPTP, mitochondrial permeability transition pore; RNAi, RNA interference; sI/R, simulated I/R; TMRM, tetramethylrhodamine methyl ester; WT, wild-type
INTRODUCTION
The Bcl-2 family members are important regulators of the mitochondrial pathway of apoptosis. These proteins determine whether the mitochondria should initiate the cell death programme and release pro-apoptotic factors such as cytochrome c. The Bcl-2 family is composed of pro- and anti-apoptotic proteins that share up to four conserved regions of BH domains (Bcl-2 homology domains) [1]. Anti-apoptotic members such as Bcl-2 and Bcl-XL contain all four subtypes of BH domains, and promote cell survival by inhibiting the function of the pro-apoptotic Bcl-2 proteins. The pro-apoptotic members can be separated into two structurally distinct subfamilies. The ‘multidomain’ proteins (Bax and Bak) share three BH regions and lack the BH4 domain. They are structurally similar to the anti-apoptotic proteins [1,2]. Studies using cells derived from knockout mice lacking both Bax and Bak have demonstrated that Bax and Bak are essential for initiation of cell death through the intrinsic pathway [3]. In contrast, BH3-only proteins, which include Bid, Bnip3, PUMA (p53 up-regulated modulator of apoptosis) and BAD, share only the BH3 domain and are structurally diverse [4]. The BH3-only proteins have been reported to initiate cell death through activation of Bax and Bak [3,5,6].
Bnip3 is a member of the BH3-only subfamily of pro-apoptotic Bcl-2 proteins based on limited homology within the BH3 domain. The BH3 domain of Bnip contains two conserved core residues, leucine at position 1 and an aspartic acid at position 6, but lacks conserved residues glycine and glutamic acid at positions 5 and 7 respectively [7]. In addition, Bnip3 does not function like previously characterized BH3-only proteins such as Bid and BAD, and previous studies have proposed that Bnip3 is part of a functionally unique subclass of BH3-only proteins. For instance, the BH3 domain of these proteins has been reported to be essential for their cell death activity, as well as for mediating heterodimerization with other Bcl-2 members [8–10]. In contrast, the BH3 domain of Bnip3 is neither required for heterodimerization with other Bcl-2 proteins nor to promote cell death [11,12]. Instead, the C-terminal transmembrane domain of Bnip3 appears to be essential for mitochondrial targeting, apoptotic function and hetero- and homo-dimerization [11,13]. In general, activation of BH3-only proteins leads to initiation of the intrinsic pathway and activation of the caspase cascade [14–16]. In contrast, Bnip3 overexpression is associated with necrotic and apoptotic cell death, as well as a massive up-regulation of autophagy [12,17–19]. Bnip3 can cause both caspase-dependent and -independent cell death [12,17,19,20].
Bnip3 has been reported to be down-regulated in pancreatic cancers [21], which correlates with resistance to chemotherapy and a worsened prognosis [22]. In contrast, Bnip3 is associated with cell death in the myocardium and is up-regulated in hearts after acute ischaemia and in a model of chronic heart failure [20,23]. Recently, we reported that Bnip3 contributes to loss of myocardial cells in I/R (ischaemia/reperfusion) by perturbation of mitochondria [19]. Although it is clear that Bnip3 exerts its action at the mitochondria, the mechanism of Bnip3-mediated cell death remains poorly defined and it is unknown whether Bnip3 requires Bax and/or Bak as downstream effectors to induce mitochondrial dysfunction. In the present study, we provide evidence that Bax and Bak play a critical role in regulating cell death by Bnip3. We demonstrate that Bnip3 causes mitochondrial dysfunction via activation of Bax or Bak, which leads to permeabilization of the outer mitochondrial membrane. Cells lacking Bax and Bak are resistant to Bnip3-mediated cytochrome c release and cell death, suggesting that these proteins are required for Bnip3-mediated cell death. Finally, we show that Bnip3-induced cell death during sI/R (simulated I/R) is mediated through activation of Bax in HL-1 myocytes.
MATERIALS AND METHODS
Cell culture and transient transfections
MEFs (mouse embryonic fibroblasts) derived from WT (wild-type) and Bax/Bak DKO (double knockout) mice, generously provided by Dr Craig B. Thompson (Department of Cancer Biology, University of Pennsylvania, Philadelphia, PA, U.S.A.), were maintained in DMEM (Dulbecco's modified Eagle's medium) supplemented with 10% (v/v) FBS (foetal bovine serum), 100 units/ml penicillin and 100 μg/ml streptomycin [5]. Atrial-derived HL-1 myocytes [24] were obtained from Dr William C. Claycomb (LSU Health Sciences Center, New Orleans, LA, U.S.A.) and cultured in Claycomb medium (JRH Biosciences) supplemented with 10% (v/v) FBS, 2 mM L-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml amphotericin B and 100 μM noradrenaline (norepinephrine) (Sigma). MEFs were transiently transfected using Lipofectamine™ 2000 (Invitrogen) and HL-1 cells were transfected with Effectene (Qiagen) according to the manufacturers' instructions.
Hypoxia and LDH (lactate dehydrogenase) release
WT and Bax/Bak DKO MEFs in growth medium without Phenol Red were deprived of oxygen in hypoxic pouches (GasPak™ EZ; BD Biosciences) at 37 °C. Cell death was assessed after 24, 48 and 72 h of hypoxia by measuring LDH release into the medium using a cytotoxicity detection kit according to the manufacturer's protocol (Roche). The released LDH is expressed as a percentage of total LDH in the well.
Immunoblotting
Cells were lysed in a buffer containing 50 mM Tris/HCl (pH 7.4), 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 1% Triton X-100 and Complete™ protease inhibitor cocktail (Roche) and cleared by centrifugation at 20000 g for 20 min. Proteins in the supernatants were separated by SDS/PAGE, transferred on to nitrocellulose, and immunoblotted with a monoclonal antibody against Bnip3 (Sigma). The protein concentrations were determined by the Coomassie Blue binding assay (Pierce) with BSA standards.
Assessment of ΔΨm (mitochondrial membrane potential) and cell death
For analysis of ΔΨm, cells were transfected with pcDNA3.1 or Bnip3 plus pEGFP (Clontech) for 24 h and then loaded with 20 nM TMRM (tetramethylrhodamine methyl ester), which only accumulates within the matrix of respiring mitochondria [25], for 20 min at 37 °C. GFP (green fluorescent protein)-positive cells were scored for TMRM fluorescence. To assess cell death, cells transfected with pcDNA3.1 or Bnip3 and MitoDsRed2 (Clontech) were assessed for permeability to YoPro-1 as previously described [19]. The percentage of cell death was determined by the ratio of YoPro-1-positive cells to MitoDsRed2-positive cells. Cells were observed through a Nikon TE300 fluorescence microscope and images of ten random fields were captured with a cooled CCD camera (charge-coupled-device camera; Orca-ER, Hamamatsu, Japan). A minimum of 200–400 transfected cells were scored from duplicate dishes in three independent experiments.
Immunofluorescence staining
Assessment of Bax and Bak conformational changes was performed as previously described [26]. Cells were transfected with pcDNA3.1 or Bnip3 and MitoDsRed2 for 24 h and then fixed with 4% (w/v) paraformaldehyde for 15 min. The cells were permeabilized with 0.1% Triton X-100 in PBS, and blocked in 5% (v/v) normal goat serum. The cells were stained with antibodies against Bax(NT) (Upstate Biotechnology), Bak(NT) (Upstate Biotechnology), cytochrome c (BD Biosciences) or Drp-1 (dynamin-related protein 1; BD Biosciences) for 1 h at 37 °C, washed three times in PBS, followed by incubation with goat anti-rabbit IgG or goat anti-mouse IgG secondary antibody coupled with Alexa Fluor® 488 (Molecular Probes). Images were captured as described above. For high-resolution microscopy, Z-stacks were acquired of cells at ×60 magnification with 0.3 μm increments and deconvolved using ten iterations of a three-dimensional blind deconvolution algorithm (AutoQuant).
sI/R
HL-1 myocytes were transfected with GFP–Bax for 48 h before being subjected to sI/R as previously described [19]. To simulate ischaemia, the cells were incubated in a buffer containing 20 mM Hepes (pH 6.6), 125 mM NaCl, 8 mM KCl, 1.2 mM KH2PO4, 1.25 mM MgSO4, 1.2 mM CaCl2, 6.25 mM NaHCO3 and 5 mM sodium lactate in hypoxic pouches (GasPak™ EZ) equilibrated with 95% N2/5% CO2 for 2 h. Reperfusion was initiated by changing to normoxic buffer containing 20 mM Hepes, pH 7.4, 110 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.25 mM MgSO4, 1.2 mM CaCl2, 25 mM NaHCO3 and 15 mM glucose, and incubating the cells at 95% O2/5% CO2 for 5 h. To down-regulate Bnip3 expression, HL-1 cells were transfected with pcDNA™6.2-GW/EmGFP-miR-Bnip378/98 or pcDNA™6.2-GW/EmGFP-miR-LacZ for 48 h prior to sI/R as previously described [19]. Cells were subjected to sI/R as described above before fixation and staining with anti-Bax(NT) and a secondary antibody coupled with Alexa Fluor® 594 (Molecular Probes). GFP-positive cells were analysed for Bax activation.
RESULTS
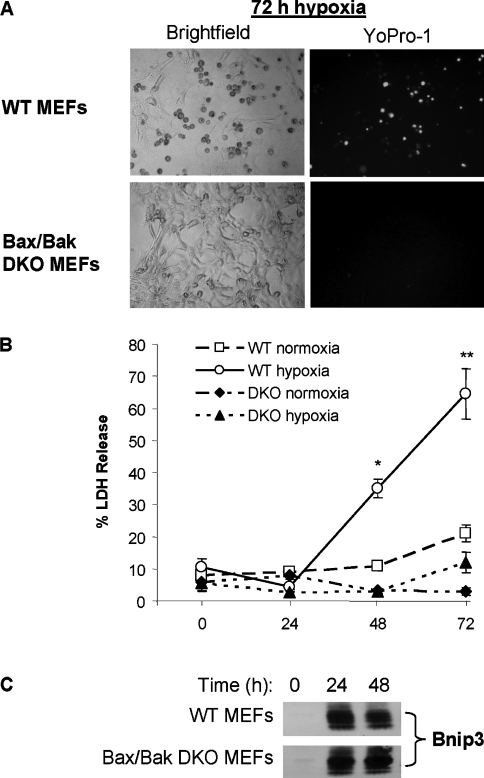
Pro-apoptotic Bcl-2 family members Bax or Bak are activated to initiate mitochondrial-mediated cell death during oxygen deprivation [27]. Since Bnip3 has been reported to be directly involved in hypoxia-mediated apoptosis [17,28], we investigated whether Bax/Bak were required for cell death during hypoxia using MEFs derived from mice lacking both Bax and Bak. WT and Bax/Bak DKO cells were deprived of oxygen for up to 72 h and cell death was assessed by measuring LDH release. We found that exposure to hypoxia resulted in a significant increase in cell death by 48 h in WT MEFs. In contrast, Bax/Bak DKO MEFs were markedly resistant to hypoxia compared with WT cells and did not show a significant increase in LDH release after up to 72 h of hypoxia (Figures 1A and 1B). Interestingly, both WT and DKO MEFs had substantially up-regulated Bnip3 protein levels after exposure to hypoxia (Figure 1C), suggesting that Bnip3 mediates cell death through activation of Bax and/or Bak during hypoxia.
Figure 1. Cells lacking Bax and Bak are resistant to hypoxia.
(A) Bright-field and YoPro-1 fluorescence microscopy of WT and Bax/Bak DKO MEFs after 72 h of hypoxia. (B) WT and Bax/Bak DKO MEFs were subjected to hypoxia and cell death was assessed by measuring LDH release as described in the Materials and methods section. (C) Bnip3 protein levels were determined by Western blot analysis. Results are means±S.E.M., n=4 (*,**P<0.05 compared with normoxia).
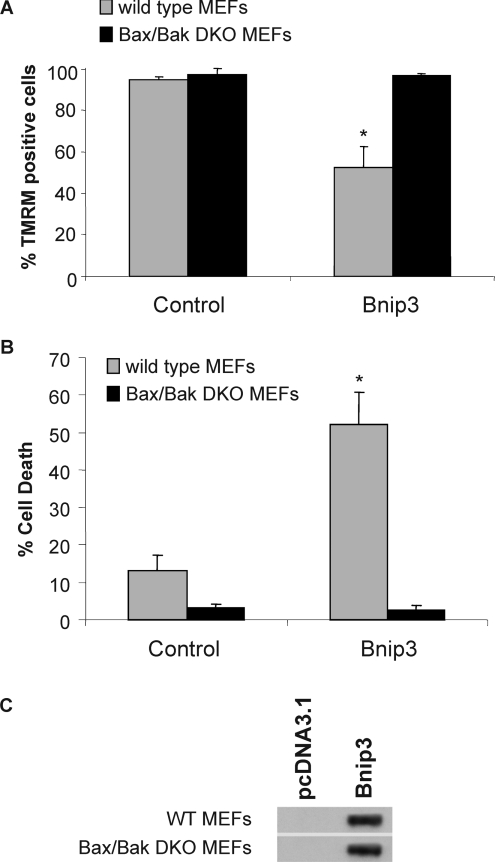
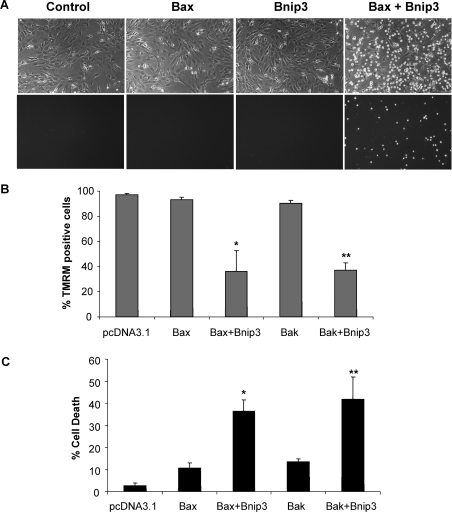
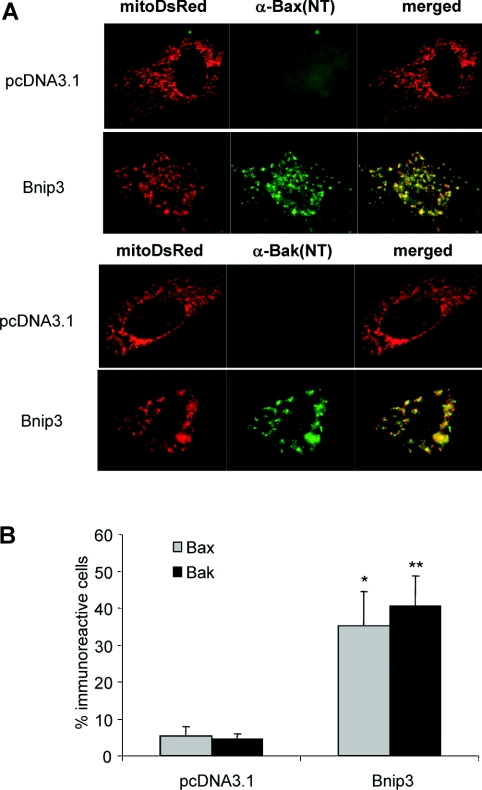
Next, we directly assessed if Bax and Bak were required for cell death induced by Bnip3. We found that overexpression of Bnip3 in WT MEFs resulted in a loss of ΔΨm and cell death, whereas DKO MEFs were completely resistant to Bnip3 overexpression (Figures 2A and 2B). Analysis of Bnip3 protein levels in transfected cells confirmed overexpression of Bnip3 in both WT and DKO MEFs (Figure 2C). To confirm that Bnip3-mediated cell death is dependent on the presence of Bax or Bak, we reintroduced Bax or Bak together with Bnip3 into Bax/Bak DKO MEFs. We found that expression of Bax or Bak in DKO MEFs restored susceptibility of these cells to Bnip3-mediated mitochondrial dysfunction and cell death (Figure 3). To confirm further that Bnip3 acts upstream of Bax and Bak, we examined whether Bnip3 induced activation of Bax and Bak in WT MEFs. We assessed activation of Bax and Bak using antibodies that specifically recognize the active conformations of Bax and Bak [29,30]. We found that overexpression of Bnip3 in WT MEFs resulted in a significant increase in immunoreactivity of both activated Bax and Bak (Figures 4A and 4B), confirming that Bnip3 can mediate mitochondrial dysfunction through activation of either Bax or Bak.
Figure 2. Cells lacking Bax and Bak are resistant to Bnip3-mediated mitochondrial dysfunction and cell death.
WT and Bax/Bak DKO MEFs were transfected with vector or Bnip3 for 24 h and (A) the ΔΨm was measured by TMRM fluorescence and (B) cell death was assessed by measuring plasma membrane permeability to YoPro-1. Results are means±S.E.M., n=3. (C) Expression of Bnip3 in WT and Bax/Bak DKO MEFs transfected with vector or Bnip3 for 24 h was determined by Western blot analysis.
Figure 3. Expression of Bax or Bak in Bax/Bak DKO MEFs restores susceptibility to Bnip3-mediated mitochondrial dysfunction and cell death.
(A) Representative bright-field (upper panel) and fluorescence (YoPro-1; lower panel) images of Bax/Bak DKO MEFs transfected with the indicated construct. (B) Significant loss of TMRM fluorescence in Bax/Bak DKO MEFs transfected with Bax or Bak together with Bnip3. (C) Bax and Bak restored Bnip3-mediated cell death as measured by permeability to YoPro-1. Results are means±S.E.M., n=3 (*,**P<0.05 compared with Bax or Bak alone).
Figure 4. Overexpression of Bnip3 activates Bax and Bak in WT MEFs.
(A) Cells co-transfected with vector or Bnip3 and MitoDsRed2 were stained with anti-Bax(NT) or anti-Bak(NT) and visualized by fluorescence microscopy. (B) Quantitative analysis of MitoDsRed2-positive cells exhibiting Bax(NT) or Bak(NT) immunoreactivity. Results are means±S.E.M., n=3 (*,**P<0.05 compared with pcDNA3.1).
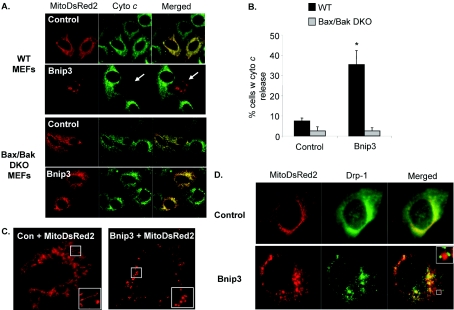
Activation of Bax or Bak results in permeabilization of the outer mitochondrial membrane and release of pro-apoptotic factors such as cytochrome c [31]. To determine whether cytochrome c release accompanies Bnip3-induced mitochondrial dysfunction, WT and Bax/Bak DKO MEFs were transfected with pcDNA3.1 or Bnip3 and the cellular distribution of cytochrome c was examined by immunofluorescence staining with an anti-(cytochrome c) antibody. As shown in Figure 5(A), WT MEFs transfected with vector had a mitochondrial distribution of cytochrome c, whereas WT MEFs overexpressing Bnip3 had released their cytochrome c from the mitochondria. In contrast, Bax/Bak DKO MEFs maintained cytochrome c in the mitochondria even in the presence of Bnip3 (Figure 5B). It has been reported that mitochondria undergo fragmentation at an early stage during apoptosis [32,33]. We found that overexpression of Bnip3 in WT MEFs caused disintegration of elongated mitochondria into numerous spherical particles (Figure 5C). We also noted that all of the cells that exhibited fragmented mitochondrial also had released their cytochrome c (results not shown). Next, we investigated whether the mitochondrial fragmentation was due to increased fission through the recruitment of Drp-1 to mitochondria. We found that Drp-1 was mainly distributed in the cytosol with some co-localization with mitochondria in control transfected cells. Drp-1 has been reported to cycle between the cytosol and the mitochondria in healthy cells [32]. In contrast, overexpression of Bnip3 caused redistribution of Drp-1 from the cytosol to the mitochondria. We also noted that Drp-1 was found in clusters on fragments of mitochondria (Figure 5D). Drp-1 did not accumulate at the mitochondria in Bax/Bak DKO in response to Bnip3 overexpression (results not shown).
Figure 5. Bnip3 induces mitochondrial fragmentation and release of cytochrome c in WT MEFs but not in Bax/Bak DKO MEFs.
(A) WT or Bax/Bak DKO MEFs were co-transfected with vector or Bnip3 and MitoDsRed2 for 24 h and then stained with an antibody towards cytochrome c and examined by immunofluorescence. Representative images are shown. (B) Quantification of cytochrome c release. Results are means±S.E.M., n=4 (*P<0.05 compared with control). (C) Overexpression of Bnip3 induces fragmentation of the mitochondrial network in WT MEFs. (D) Recruitment of Drp-1 to mitochondria in WT MEFs overexpressing Bnip3.
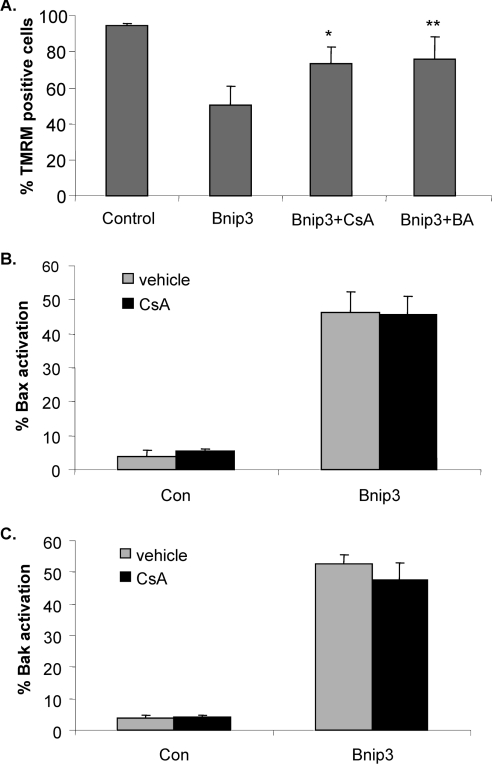
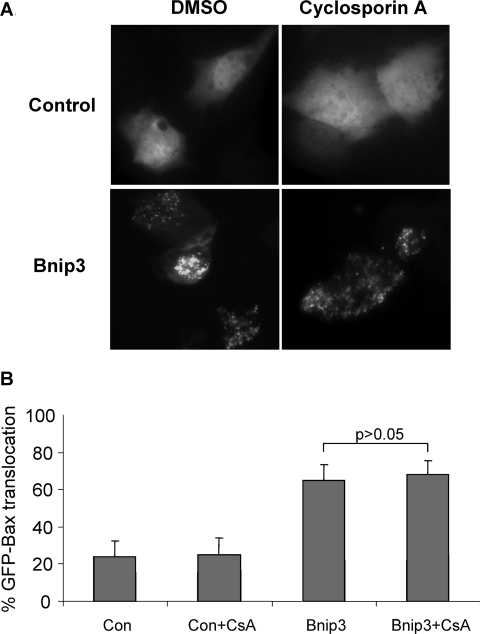
Several studies have reported that Bnip3 mediates cell death through opening of the mPTP (mitochondrial permeability transition pore) in different cell types [12,17,20]. Similarly, we found that treatment of WT MEFs overexpressing Bnip3 with CsA (cyclosporin A) or BA (bongkrekic acid), two different inhibitors of the mPTP, partially protected against Bnip3-mediated mitochondrial dysfunction, confirming that Bnip3-induced loss of ΔΨm is due, in part, whether opening of the mPTP (Figure 6A). Since mPTP opening has been reported to stimulate translocation of Bax from the cytosol to the mitochondria, we investigated whether opening of the mPTP by Bnip3 caused activation of Bax and Bak. WT MEFs were transfected with vector or Bnip3 plus MitoDsRed2 in the presence or absence of CsA for 24 h before activation of Bax and Bak were assessed. Interestingly, inhibition of mPTP did not prevent activation of Bax and Bak by Bnip3 in WT MEFs (Figures 6B and 6C). To investigate further the role of the mPTP in Bnip3-mediated activation of Bax, Bax/Bak DKO MEFs were transfected with vector or Bnip3 plus GFP–Bax and localization of GFP–Bax was assessed by immunofluorescence microscopy. In control transfected cells, GFP–Bax was homogeneously distributed throughout the cytosol, whereas GFP–Bax accumulated at the mitochondria in cells overexpressing Bnip3 (Figure 7A). Treatment with CsA did not inhibit translocation of GFP–Bax to the mitochondria in Bax/Bak DKO MEFs overexpressing Bnip3, confirming that opening of the mPTP by Bnip3 does not trigger activation of Bax or Bak (Figure 7B).
Figure 6. Inhibition of the mPTP reduces Bnip3-mediated mitochondrial dysfunction, but does not prevent activation of Bax and Bak.
(A) WT MEFs transfected with vector or Bnip3 were treated with 2 μM CsA or 100 μM BA and stained with TMRM to assess ΔΨm after 24 h (*,**P<0.05 compared with Bnip3 alone). WT MEFs transfected with vector or Bnip3 and treated with CsA were fixed after 24 h and stained with (B) anti-Bax(NT) or (C) anti-Bak(NT). Results are means±S.E.M., n=3.
Figure 7. Inhibition of mPTP opening with CsA does not prevent Bnip3-induced translocation of GFP–Bax to mitochondria.
(A) Bax/Bak DKO MEFs were co-transfected with vector or Bnip3 plus GFP–Bax and then treated with CsA for 24 h before being analysed by fluorescence microscopy. Images shown are representative of results obtained in three separate experiments. (B) Quantification of the percentage cells with either diffuse or mitochondrial GFP–Bax fluorescence. Results are means±S.E.M., n=3.
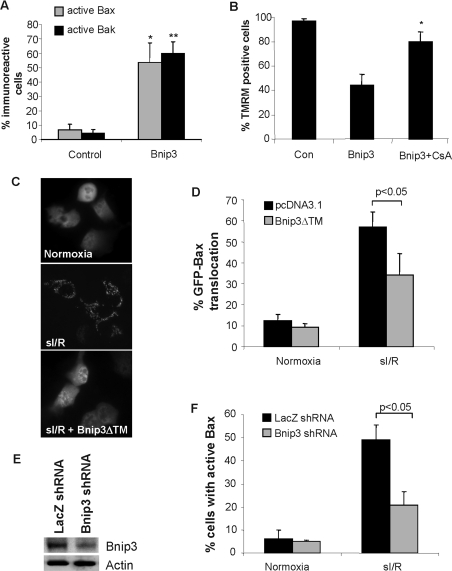
Bcl-2 proteins are important regulators of apoptosis in the myocardium and we have previously found that Bnip3 contributes to myocardial I/R injury [19]. Bnip3 overexpression directly activated Bax and Bak in HL-1 myocytes (Figure 8A), and treatment with CsA significantly preserved ΔΨm in Bnip3-overexpressing cells (Figure 8B), confirming that Bnip3 causes mitochondrial dysfunction through a similar mechanism in HL-1 myocytes and MEFs. To investigate whether Bnip3 causes cell death during I/R through activation of Bax, we examined the effects of the dominant-negative Bnip3, Bnip3ΔTM [17,19,20], on Bax translocation to the mitochondria in response to sI/R in HL-1 myocytes. We found that cells transfected with GFP–Bax had predominantly a diffuse distribution of green fluorescence during normoxic conditions. Exposure of cells to sI/R resulted in translocation of GFP–Bax to mitochondria, whereas overexpression of Bnip3ΔTM significantly reduced translocation of GFP–Bax during sI/R (Figures 8C and 8D). The role of Bnip3 in inducing Bax activation during sI/R was confirmed by down-regulation of Bnip3 by RNAi (RNA interference). Transfection of cells with RNAi against Bnip3 resulted in a 70–80% reduction in Bnip3 protein levels (Figure 8E) and resulted in reduced activation of Bax during sI/R (Figure 8F).
Figure 8. Bnip3 mediates cell death through activation of Bax during sI/R in HL-1 myocytes.
(A) Overexpression of Bnip3 activates Bax and Bak in HL-1 myocytes. Cells were transfected with vector or Bnip3 plus MitoDsRed2 and stained with anti-Bax(NT) or anti-Bak(NT). Results are means±S.E.M., n=3. (*,**P<0.05 compared with control). (B) Treatment with CsA to inhibit the mPTP decreases Bnip3-mediated loss of ΔΨm as measured by loss of TMRM fluorescence. Results are means±S.E.M., n=3 (*P<0.05 compared with Bnip3 alone). (C) HL-1 myocytes were transfected with pcDNA3.1 or Bnip3ΔTM plus GFP–Bax for 48 h and then subjected to 2 h of simulated ischaemia and 5 h of reperfusion. GFP–Bax distribution was assessed by fluorescence microscopy. Images shown are from a representative experiment. (D) Quantification of GFP–Bax distribution in HL-1 myocytes. Results are means±S.E.M., n=3. (E) Cells were transfected with a vector encoding RNAi against either Bnip3 or LacZ, and cell lysates were analysed for Bnip3 expression by Western blotting after 48 h of expression. (F) Down-regulation of Bnip3 by RNAi reduces sI/R-stimulated translocation of GFP–Bax to the mitochondria. Results are means±S.E.M., n=3.
DISCUSSION
Bnip3 is a critical regulator of mitochondrial function in myocardial cells and has been implicated in myocardial ischaemia and heart failure. We have previously shown that Bnip3 contributes to myocardial I/R injury and that blocking Bnip3 with Bnip3ΔTM through transactivator-mediated protein transduction can preserve mitochondrial integrity and reduce the incidence of cell death after I/R [19]. In the present study, we have investigated the molecular mechanism by which Bnip3 mediates the loss of ΔΨm and cell death. We provide evidence that pro-apoptotic Bax and Bak play a critical role in regulating mitochondrial dysfunction by Bnip3. Our results also confirm that Bnip3 mediates I/R injury through activation of Bax in HL-1 cardiac myocytes. To our knowledge, this is the first study to demonstrate that Bnip3 mediates mitochondrial dysfunction and cell death via Bax and Bak.
Bnip3 is known to localize to and exert its action at the mitochondria. Since Bax and Bak have been shown to be key regulators of the mitochondrial pathway of apoptosis, it seemed likely that Bnip3 would initiate cell death via Bax and/or Bak. Our results clearly demonstrate that Bax and Bak play a role in Bnip3-mediated mitochondrial dysfunction. First, cells lacking Bax and Bak were completely resistant to hypoxia even though Bnip3 was substantially up-regulated in these cells. Secondly, overexpression of Bnip3 caused a rapid loss of ΔΨm in WT cells, whereas Bax/Bak DKO cells were resistant to Bnip3. Reintroduction of Bax or Bak restored sensitivity to Bnip3, suggesting that Bax and Bak have overlapping functions in Bnip3-mediated cell death. Finally, overexpression of Bnip3 induced activation of Bax and Bak, which correlated with permeabilization of the outer mitochondrial membrane in WT MEFs.
Our results show that Bnip3 induces activation of Bax and Bak, but it is not clear exactly how Bnip3 activates Bax and Bak. BH3-only proteins have been reported to induce activation of Bax and Bak by direct interaction or indirectly by sequestering anti-apoptotic members such as Bcl-2 and Bcl-XL [34–36]. Bnip3 and Bak are both constitutively localized to the mitochondria and could potentially form heterodimers. However, Bax is localized to the cytosol under normal conditions and only translocates to the mitochondria in response to stress and therefore is not likely to require direct interaction with Bnip3 for activation. Instead, Bnip3 has been shown to directly interact with Bcl-2 and Bcl-XL, suggesting that Bnip3 could activate Bax and Bak through an indirect mechanism by sequestering Bcl-2 and Bcl-XL. The mPTP has been reported to play a role in the execution of the mitochondrial apoptotic programme and many studies have reported a functional link between Bax and the mPTP. There are reports of the mPTP regulating Bax in apoptosis, where opening of the mPTP stimulates Bax translocation to mitochondria [37,38]. Several studies have reported that Bnip3-mediated cell death occurs through opening of the mPTP [12,17,20], and our present results confirm that Bnip3-induced mitochondrial dysfunction is mediated through the mPTP. However, our results show clearly that Bnip3-mediated opening of the mPTP does not cause activation of Bax and Bak. This suggests that opening of the mPTP occurs downstream of Bax/Bak activation or possibly by a separate pathway activated by Bnip3.
DNA fragmentation and chromatin condensation are characteristics of caspase-dependent cell death and are usually observed in cells overexpressing Bnip3 [17,20,39]. Our results demonstrate that Bax or Bak is essential for Bnip3-mediated mitochondrial dysfunction and cell death. However, Bnip3 has been reported to induce both caspase-dependent and -independent cell death. Although activation of Bax and Bak triggers the release of cytochrome c and activation of caspases, activation of Bax and Bak also initiates caspase-independent mitochondrial dysfunction [6,40,41]. For instance, Cheng et al. [6] reported that overexpression of BH3-only proteins BAD, Bim (Bcl-2-interacting mediator of cell death) or Noxa induced mitochondrial dysfunction and caspase-independent cell death via Bax and Bak in cells lacking Apaf-1 (apoptotic protease-activating factor-1), caspase 9 or caspase 3. Bax and Bak have been reported to initiate apoptosis through activation of the mitochondrial fission pathway. Both Bax and Bak were reported to co-localize with the fission protein Drp-1 at defined foci on the mitochondrial membrane at the onset of apoptosis [32,33]. Bax and Bak were also shown to induce caspase-independent cell death by the release of DDP/TIMM8α, a factor that is involved in mitochondrial recruitment of Drp-1, from the mitochondria [42]. Thus it is possible that Bnip3 via Bax and Bak mediates caspase-independent cell death through the mitochondrial fission pathway. Taken together, these results are consistent with the notion that BH3-only proteins, including Bnip3, require Bax and/or Bak as downstream effectors to initiate the apoptotic programme, but that the downstream mechanisms of cell death may be caspase-dependent or -independent.
The pro-apoptotic Bcl-2 proteins have been implicated in the pathogenesis of various cardiac diseases, including myocardial infarction and heart failure. We have found that Bnip3 is a major contributor to myocardial I/R injury [19] and, in the present study, we provide further evidence that one of the upstream activators of Bax in I/R is Bnip3. Bax has been reported to be activated in cardiac cells in response to oxidative stress [26] and during I/R [37]. Hearts from Bax-deficient mice had reduced mitochondrial damage and decreased infarct size after I/R compared with WT, implicating Bax as a major player in I/R injury [43]. In conclusion, the pro-apoptotic Bcl-2 proteins, including Bnip3, may be important potential therapeutic targets for treatment and prevention of myocardial I/R injury to limit loss of contractile cells.
Acknowledgments
We thank Dr Craig B. Thompson for providing us with WT and Bax/Bak DKO MEFs and Dr William C. Claycomb for the HL-1 cells. We also thank Dr Lorrie A. Kirshenbaum (Institute of Cardiovascular Science, University of Manitoba, Winnipeg, Manitoba, Canada) for providing Bnip3 and Bnip3ΔTM, and Dr Richard Youle [NIH (National Institutes of Health), Bethesda, MD, U.S.A.] for pEGFP-Bax. This research was supported by funds from the Biomedical Post-Baccalaureate Research Education Program (to J. E. Y.), NIH grant number GM 64104, the California Tobacco-Related Disease Research Program of the University of California, grant number 14KT-0109 (to Å. B. G.), and the Stein Endowment Fund.
References
- 1.Adams J. M., Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki M., Youle R. J., Tjandra N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell. 2000;103:645–654. doi: 10.1016/s0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
- 3.Wei M. C., Zong W. X., Cheng E. H., Lindsten T., Panoutsakopoulou V., Ross A. J., Roth K. A., MacGregor G. R., Thompson C. B., Korsmeyer S. J. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang D. C., Strasser A. BH3-only proteins-essential initiators of apoptotic cell death. Cell. 2000;103:839–842. doi: 10.1016/s0092-8674(00)00187-2. [DOI] [PubMed] [Google Scholar]
- 5.Zong W. X., Lindsten T., Ross A. J., MacGregor G. R., Thompson C. B. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 2001;15:1481–1486. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng E. H., Wei M. C., Weiler S., Flavell R. A., Mak T. W., Lindsten T., Korsmeyer S. J. BCL-2, BCL-XL sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol. Cell. 2001;8:705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 7.Yasuda M., Theodorakis P., Subramanian T., Chinnadurai G. Adenovirus E1B-19K/BCL-2 interacting protein BNIP3 contains a BH3 domain and a mitochondrial targeting sequence. J. Biol. Chem. 1998;273:12415–12421. doi: 10.1074/jbc.273.20.12415. [DOI] [PubMed] [Google Scholar]
- 8.Boyd J. M., Gallo G. J., Elangovan B., Houghton A. B., Malstrom S., Avery B. J., Ebb R. G., Subramanian T., Chittenden T., Lutz R. J. Bik, a novel death-inducing protein shares a distinct sequence motif with Bcl-2 family proteins and interacts with viral and cellular survival-promoting proteins. Oncogene. 1995;11:1921–1928. [PubMed] [Google Scholar]
- 9.Chittenden T., Flemington C., Houghton A. B., Ebb R. G., Gallo G. J., Elangovan B., Chinnadurai G., Lutz R. J. A conserved domain in Bak, distinct from BH1 and BH2, mediates cell death and protein binding functions. EMBO J. 1995;14:5589–5596. doi: 10.1002/j.1460-2075.1995.tb00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang K., Yin X. M., Chao D. T., Milliman C. L., Korsmeyer S. J. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 11.Ray R., Chen G., Vande V. C., Cizeau J., Park J. H., Reed J. C., Gietz R. D., Greenberg A. H. BNIP3 heterodimerizes with Bcl-2/Bcl-XL and induces cell death independent of a Bcl-2 homology 3 (BH3) domain at both mitochondrial and nonmitochondrial sites. J. Biol. Chem. 2000;275:1439–1448. doi: 10.1074/jbc.275.2.1439. [DOI] [PubMed] [Google Scholar]
- 12.Vande V. C., Cizeau J., Dubik D., Alimonti J., Brown T., Israels S., Hakem R., Greenberg A. H. BNIP3 and genetic control of necrosis-like cell death through the mitochondrial permeability transition pore. Mol. Cell. Biol. 2000;20:5454–5468. doi: 10.1128/mcb.20.15.5454-5468.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen G., Ray R., Dubik D., Shi L., Cizeau J., Bleackley R. C., Saxena S., Gietz R. D., Greenberg A. H. The E1B 19K/Bcl-2-binding protein Nip3 is a dimeric mitochondrial protein that activates apoptosis. J. Exp. Med. 1997;186:1975–1983. doi: 10.1084/jem.186.12.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hegde R., Srinivasula S. M., Ahmad M., Fernandes-Alnemri T., Alnemri E. S. Blk, a BH3-containing mouse protein that interacts with Bcl-2 and Bcl-xL, is a potent death agonist. J. Biol. Chem. 1998;273:7783–7786. doi: 10.1074/jbc.273.14.7783. [DOI] [PubMed] [Google Scholar]
- 15.Luo X., Budihardjo I., Zou H., Slaughter C., Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 16.Oda E., Ohki R., Murasawa H., Nemoto J., Shibue T., Yamashita T., Tokino T., Taniguchi T., Tanaka N. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288:1053–1058. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- 17.Kubasiak L. A., Hernandez O. M., Bishopric N. H., Webster K. A. Hypoxia and acidosis activate cardiac myocyte death through the Bcl-2 family protein BNIP3. Proc. Natl. Acad. Sci. U.S.A. 2002;99:12825–12830. doi: 10.1073/pnas.202474099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daido S., Kanzawa T., Yamamoto A., Takeuchi H., Kondo Y., Kondo S. Pivotal role of the cell death factor BNIP3 in ceramide-induced autophagic cell death in malignant glioma cells. Cancer Res. 2004;64:4286–4293. doi: 10.1158/0008-5472.CAN-03-3084. [DOI] [PubMed] [Google Scholar]
- 19.Hamacher-Brady A., Brady N. R., Logue S. E., Sayen M. R., Jinno M., Kirshenbaum L. A., Gottlieb R. A., Gustafsson A. B. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ. 2007;14:146–157. doi: 10.1038/sj.cdd.4401936. [DOI] [PubMed] [Google Scholar]
- 20.Regula K. M., Ens K., Kirshenbaum L. A. Inducible expression of BNIP3 provokes mitochondrial defects and hypoxia-mediated cell death of ventricular myocytes. Circ. Res. 2002;91:226–231. doi: 10.1161/01.res.0000029232.42227.16. [DOI] [PubMed] [Google Scholar]
- 21.Okami J., Simeone D. M., Logsdon C. D. Silencing of the hypoxia-inducible cell death protein BNIP3 in pancreatic cancer. Cancer Res. 2004;64:5338–5346. doi: 10.1158/0008-5472.CAN-04-0089. [DOI] [PubMed] [Google Scholar]
- 22.Erkan M., Kleeff J., Esposito I., Giese T., Ketterer K., Buchler M. W., Giese N. A., Friess H. Loss of BNIP3 expression is a late event in pancreatic cancer contributing to chemoresistance and worsened prognosis. Oncogene. 2005;24:4421–4432. doi: 10.1038/sj.onc.1208642. [DOI] [PubMed] [Google Scholar]
- 23.Graham R. M., Frazier D. P., Thompson J. W., Haliko S., Li H., Wasserlauf B. J., Spiga M. G., Bishopric N. H., Webster K. A. A unique pathway of cardiac myocyte death caused by hypoxia–acidosis. J. Exp. Biol. 2004;207:3189–3200. doi: 10.1242/jeb.01109. [DOI] [PubMed] [Google Scholar]
- 24.Claycomb W. C., Lanson N. A., Jr, Stallworth B. S., Egeland D. B., Delcarpio J. B., Bahinski A., Izzo N. J., Jr HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc. Natl. Acad. Sci. U.S.A. 1998;95:2979–2984. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernardi P., Scorrano L., Colonna R., Petronilli V., Di Lisa F. Mitochondria and cell death. Mechanistic aspects and methodological issues. Eur. J. Biochem. 1999;264:687–701. doi: 10.1046/j.1432-1327.1999.00725.x. [DOI] [PubMed] [Google Scholar]
- 26.Gustafsson A. B., Tsai J. G., Logue S. E., Crow M. T., Gottlieb R. A. Apoptosis repressor with caspase recruitment domain protects against cell death by interfering with Bax activation. J. Biol. Chem. 2004;279:21233–21238. doi: 10.1074/jbc.M400695200. [DOI] [PubMed] [Google Scholar]
- 27.Brunelle J. K., Chandel N. S. Oxygen deprivation induced cell death: an update. Apoptosis. 2002;7:475–482. doi: 10.1023/a:1020668923852. [DOI] [PubMed] [Google Scholar]
- 28.Kothari S., Cizeau J., Millan-Ward E., Israels S. J., Bailes M., Ens K., Kirshenbaum L. A., Gibson S. B. BNIP3 plays a role in hypoxic cell death in human epithelial cells that is inhibited by growth factors EGF and IGF. Oncogene. 2003;22:4734–4744. doi: 10.1038/sj.onc.1206666. [DOI] [PubMed] [Google Scholar]
- 29.Desagher S., Osen-Sand A., Nichols A., Eskes R., Montessuit S., Lauper S., Maundrell K., Antonsson B., Martinou J. C. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zong W. X., Li C., Hatzivassiliou G., Lindsten T., Yu Q. C., Yuan J., Thompson C. B. Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J. Cell Biol. 2003;162:59–69. doi: 10.1083/jcb.200302084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Danial N. N., Korsmeyer S. J. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 32.Frank S., Gaume B., Bergmann-Leitner E. S., Leitner W. W., Robert E. G., Catez F., Smith C. L., Youle R. J. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev. Cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- 33.Karbowski M., Lee Y. J., Gaume B., Jeong S. Y., Frank S., Nechushtan A., Santel A., Fuller M., Smith C. L., Youle R. J. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J. Cell Biol. 2002;159:931–938. doi: 10.1083/jcb.200209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuwana T., Bouchier-Hayes L., Chipuk J. E., Bonzon C., Sullivan B. A., Green D. R., Newmeyer D. D. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol. Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 35.Letai A., Bassik M. C., Walensky L. D., Sorcinelli M. D., Weiler S., Korsmeyer S. J. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 36.Kelekar A., Chang B. S., Harlan J. E., Fesik S. W., Thompson C. B. Bad is a BH3 domain-containing protein that forms an inactivating dimer with Bcl-XL. Mol. Cell. Biol. 1997;17:7040–7046. doi: 10.1128/mcb.17.12.7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brady N. R., Hamacher-Brady A., Gottlieb R. A. Proapoptotic BCL-2 family members and mitochondrial dysfunction during ischemia/reperfusion injury, a study employing cardiac HL-1 cells and GFP biosensors. Biochim. Biophys. Acta. 2006;1757:667–678. doi: 10.1016/j.bbabio.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 38.Precht T. A., Phelps R. A., Linseman D. A., Butts B. D., Le S. S., Laessig T. A., Bouchard R. J., Heidenreich K. A. The permeability transition pore triggers Bax translocation to mitochondria during neuronal apoptosis. Cell Death Differ. 2005;12:255–265. doi: 10.1038/sj.cdd.4401552. [DOI] [PubMed] [Google Scholar]
- 39.Guo K., Searfoss G., Krolikowski D., Pagnoni M., Franks C., Clark K., Yu K. T., Jaye M., Ivashchenko Y. Hypoxia induces the expression of the pro-apoptotic gene BNIP3. Cell Death Differ. 2001;8:367–376. doi: 10.1038/sj.cdd.4400810. [DOI] [PubMed] [Google Scholar]
- 40.Fitch M. E., Chang C. M., Parslow T. G. The BH3 domain is required for caspase-independent cell death induced by Bax and oligomycin. Cell Death Differ. 2000;7:338–349. doi: 10.1038/sj.cdd.4400659. [DOI] [PubMed] [Google Scholar]
- 41.Xiang J., Chao D. T., Korsmeyer S. J. BAX-induced cell death may not require interleukin 1β-converting enzyme-like proteases. Proc. Natl. Acad. Sci. U.S.A. 1996;93:14559–14563. doi: 10.1073/pnas.93.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arnoult D., Rismanchi N., Grodet A., Roberts R. G., Seeburg D. P., Estaquier J., Sheng M., Blackstone C. Bax/Bak-dependent release of DDP/TIMM8a promotes Drp1-mediated mitochondrial fission and mitoptosis during programmed cell death. Curr. Biol. 2005;15:2112–2118. doi: 10.1016/j.cub.2005.10.041. [DOI] [PubMed] [Google Scholar]
- 43.Hochhauser E., Kivity S., Offen D., Maulik N., Otani H., Barhum Y., Pannet H., Shneyvays V., Shainberg A., Goldshtaub V., et al. Bax ablation protects against myocardial ischemia-reperfusion injury in transgenic mice. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H2351–H2359. doi: 10.1152/ajpheart.00783.2002. [DOI] [PubMed] [Google Scholar]