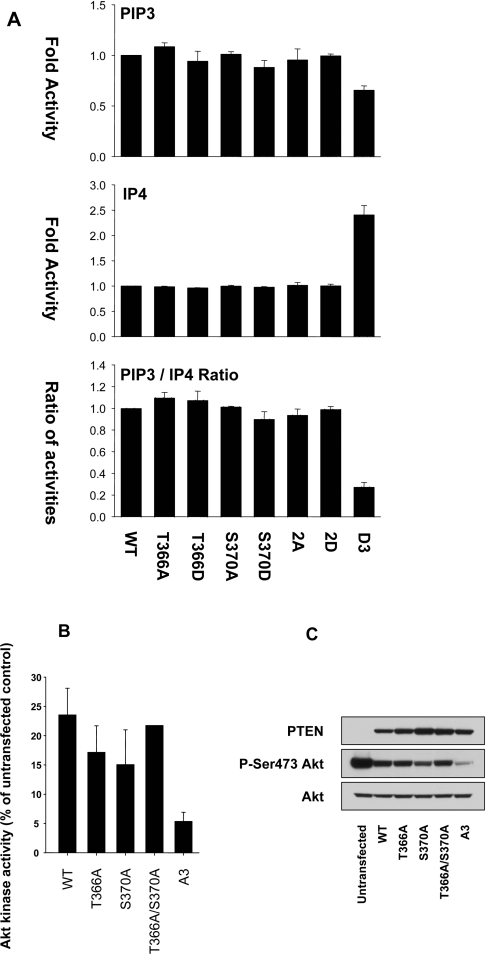
Figure 3. The PTEN phosphorylation sites Thr366 and Ser370 do not affect phosphatase activity in vitro or in cells.
(A) Bacterially expressed PTEN proteins were assayed in vitro against phosphatidylcholine/PtdIns(3,4,5)P3 vesicles (100 ng of enzyme) and against the soluble substrate Ins(1,3,4,5)P4 (500 ng of enzyme). PTEN 2A and 2D refer to double mutants with either alanine or aspartic acid replacements respectively, of both Thr366 and Ser370. PTEN D3 refers to an aspartic acid triple replacement mutant of Ser380, Thr382 and Thr383. Results are shown for each substrate as the mean±S.E.M. activity from triplicate samples and also the ratio of these two activities, shown as the mean±S.E.M. ratio from these paired triplicate samples. (B, C) U87MG cells were infected with viruses encoding the indicated PTEN proteins. PTEN A3 refers to an alanine triple replacement mutant of Ser380, Thr382 and Thr383. At 24 h after infection, cells were lysed and the activity (B) and phosphorylation (C) of endogenous Akt/PKB was determined by immunoprecipitate kinase assay and Western blotting respectively. WT, wild-type.