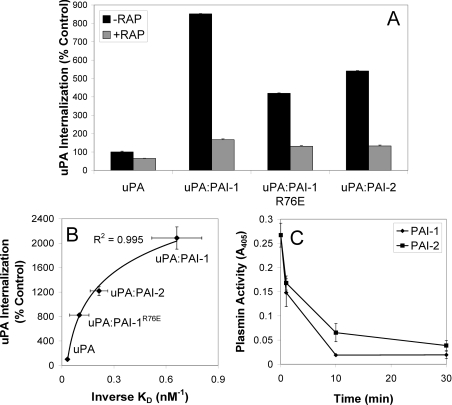
Figure 4. VLDLr-mediated internalization of uPA–serpin complexes by MCF-7 cells.
(A) MCF-7 cells were incubated in the presence or absence of RAP (200 nM) for 15 min at 37 °C, prior to analysis of uPA–Alexa Fluor® 488 internalization using a fluorescence quenching internalization assay (means±S.E.M., n=3). (B) Relationship between the affinity of uPA and uPA–serpin complexes for VLDLr (Table 1, n=3) and internalization (n=2). (C) The inhibition of cell surface uPA activity by PAI-1 and PAI-2. MCF-7 cells pre-incubated with uPA (5 nM) at 4 °C for 30 min were washed and then incubated with PAI-1 or PAI-2 (5 nM) at 37 °C for the time periods indicated. uPA activity was measured indirectly by the addition of plasminogen (0.5 μM) and Spectrozyme-PL (0.4 mM) for 2 h. Absorbance was read at 405 nm. Activity in the absence of exogenous uPA was subtracted from all measurements (means±S.E.M., n=3).