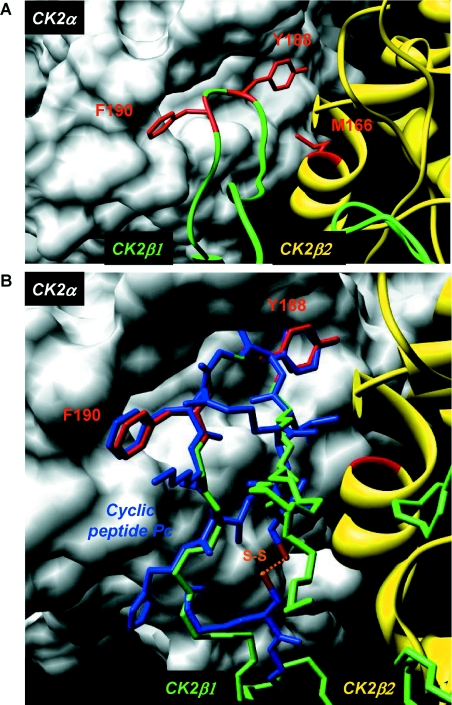
Figure 3. Structure and mode of binding of Pc peptide on CK2α.
(A) Surface representation of the binding pocket of CK2α interacting with a short C-terminal loop of CK2β. Crystal structure of the CK2 holoenzyme shows that a C-terminal fragment of the CK2β1 chain encompassing residues 186–193 (green) forms a loop inserting into a deep hydrophobic pocket of CK2α [14]. Surface representation of the CK2α cleft in grey highlights its pocket-like characteristics. The phenyl and phenol rings of the two non-polar and aromatic CK2β residues Tyr188 and Phe190 respectively (red) are in quasi-planar opposite orientation and fit tightly into it. Met166 (red) located on the second CK2β2 chain (yellow) is labelled. (B) Molecular model of Pc peptide in CK2α pocket. A rough molecular model of Pc peptide (blue) was obtained from the CK2β1 structure determined previously (PDB code 1JWH [13]). In detail, the CK2β1 structure segment (R186LYGFKIH193 in green) was taken as template for the corresponding central peptide segment, and the terminal amino acids were added manually using the program Coot [39]. A basic geometric idealization of peptide bonds was then performed using the program Refmac 5 [40] from CCP4 [41]. For clarity, only key interacting residues of CK2β1 are shown (in red). The cysteine disulfide bridge is coloured orange. The superimposition of Pc peptide with the CK2β C-terminal loop demonstrates that Pc emulates the interaction of the natural ligand CK2β with CK2α. Figures were prepared with the Chimera software [42] and the 1JWH PDB structure [14].