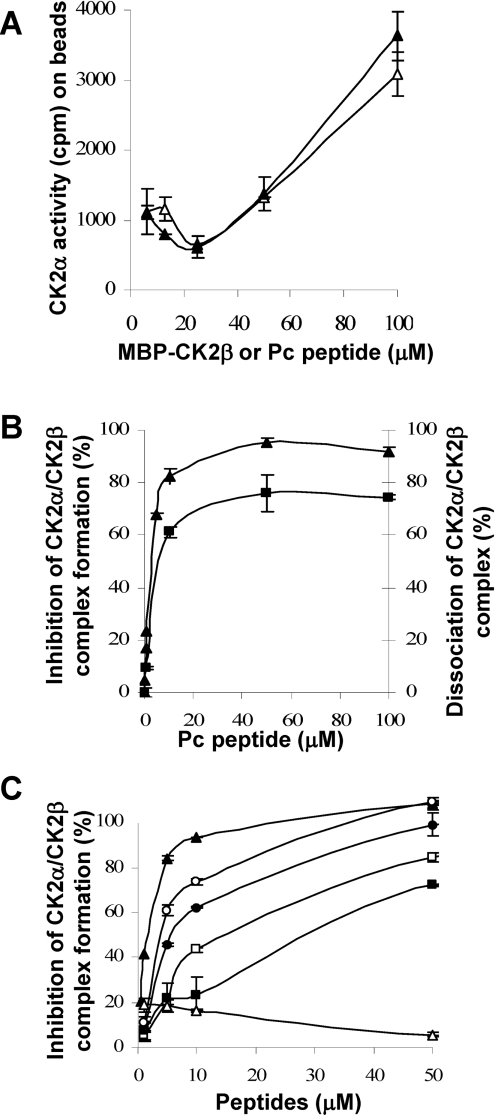
Figure 7. Disruption of CK2 subunit interaction by CK2β-derived cyclic peptides.
(A) Binding of Pc peptide or CK2β to CK2α. Increasing concentrations of biotinylated Pc peptide (▲) or biotinylated MBP–CK2β (Δ) were immobilized on streptavidin beads and incubated with CK2α (0.3 μM) for 15 min at 4 °C. After washing, the amount of associated CK2α was evaluated by a CK2 kinase assay. (B) Inhibition of CK2α–CK2β complex formation by Pc peptide (▲). Immobilized MBP–CK2β was incubated with increasing concentrations of Pc peptide, followed by the addition of [35S]methionine-labelled CK2α (105 c.p.m.). After washing, bound CK2α was determined by radioactivity counting. Dissociation of pre-formed CK2α–CK2β complex by Pc peptide (■). [35S]Methionine-labelled CK2α was incubated with immobilized MBP–CK2β, followed by the addition of increasing concentrations of Pc peptide. After washing, bound CK2α was determined by radioactivity counting. (C) Inhibition of CK2α–CK2β complex formation by different Pc peptide derivatives. Increasing concentrations of the different peptides were tested as in (B). Pc (▲), linear peptide GAGGRLYGFKIHGGP (■), inverted linear peptide PGGHIKFGYLRGGAG (Δ), head-to-tail peptide analogues: PcL1 RLYGFKIHPGAGG (□), PcL2 RLYGFKIHGPGAGG (●) and PcL3 RLYGFKIHGGPGAGG (○). Results are the means±S.D. (n=3).