Abstract
Cholesterol ester transfer protein (CETP) plays a key role in remodeling triglyceride-rich particles and high-density lipoproteins (HDL). We investigated CETP sequence variants in response to long-term overfeeding (100 days) in 12 pairs of male monozygotic twins (mean age±SD: 21±2 y). Body fat mass (FM), abdominal subcutaneous (ASF) and visceral fat (AVF), and plasma lipoproteins were determined. The CETP variants C>T/In9 (rs289714) and G>A/Ex14 (rs5882, or I405V) were investigated by RFLP-PCR methodologies. Before overfeeding, the CETP CC/In9 (n=18) genotype was associated with lower FM compared to the C>T/In9 heterozygotes. Overfeeding induced more FM and ASF accretion in C>T/In9 carriers (P ≤ 0.05). CETP V405V homozygotes (n=8) had lower BMI, FM, and ASF before overfeeding than those with the I405I (n=6) or I405V (n=10) genotypes. However, V405V subjects had the largest gain in AVF with overfeeding (P = 0.02). Decreases from baseline were significantly different across the I405V genotypes for HDL-C, HDL-Apo AI, HDL2, and HDL3 (P ≤ 0.05). Our data suggests that CETP sequence variation contributes to the undesirable changes in adiposity and HDL-C levels when exposed to excessive calorie consumption and may be potentially helpful to identify individuals with the metabolic syndrome who are at higher risk of cardiovascular disease.
Keywords: Adiposity, obesity, lipoprotein, overfeeding, CETP, HDL
INTRODUCTION
Polymorphisms in specific genes are increasingly implicated in the response to dietary challenges, particularly to excess calories and in the obese state, which are associated with a high risk of coronary heart disease (CHD)1. High-density lipoprotein cholesterol (HDL-C), is partly regulated by cholesteryl ester transfer protein (CETP).
CETP mediates the exchange of TG for cholesterol esters (CE)2. Synthesized mainly in the liver, it circulates bound to HDL containing Apo A1 particles3. CETP mRNA and protein concentration rise in response to high cholesterol levels and dietary fat intake, increase the transfer of CE to HDL particles 4, 5, enhance the return of cholesterol from the plasma to the liver, and decrease plasma cholesterol levels. CETP is anti-atherogenic, when associated to high HDL levels 6, while CETP deficiency diminishes cellular cholesterol efflux and, with low HDL-C levels, promotes atherosclerosis 7, 8.
CETP polymorphisms have been related to variations in plasma HDL-C 9, 10. Environmental factors influence CETP plasma levels 11–14 but no data, to our knowledge, have been reported on the effects of CETP polymorphisms in chronic dietary energy surplus. This study evaluated the effects of selected CETP polymorphisms on adiposity and lipoprotein changes in response to a long-term overfeeding protocol.
METHODS
The study design and methodology of this overfeeding protocol have already been published 15. Only a brief description will be presented here.
Subjects
Twenty-four sedentary men (12 pairs of monozygotic twins), aged between 19 and 27 years (mean age ± SD: 21.0 ± 2.0 years), gave their written informed consent to participate in this study, which received the approval of the Medical Ethics Committee of Laval University and the Office for the Protection from Research Risk of the National Institutes of Health. Participants were subjected to a full physical examination and those with overweight, cardiovascular disease, diabetes, dyslipoproteinemia, hypertension, or endocrine disorders excluded.
Measurement of baseline energy needs
Twins were studied in three subgroups of four pairs each, under 24-hour close supervision by the research staff. Subjects lived on Laval University campus for 120 consecutive days that included 2 weeks of baseline measurements, 3 days for testing before overfeeding, and 3 days for testing after the 100-day overfeeding protocol. Body weight, skinfold measurements, and body composition assessments were used to monitor changes in body mass or composition. Individual energy needs were evaluated during the first 14 days of observation. All foods consumed by the subjects during this period were carefully weighed and recorded. The nutrient composition and caloric intake were derived from Canadian food composition tables 16.
Overfeeding protocol
Following the baseline monitoring period and the pre-overfeeding measurements, subjects were overfed by 4.2 MJ (1000 kcal) per day above their individually measured energy requirements, 6 days per week, for a period of 100 days. One day each week, they were not overfed but were fed the amount of energy determined during the baseline observation period, for a total energy surplus of 353 MJ (84,000 kcal). During the entire project, subjects were kept sedentary. As they lived together under the same supervised conditions, substantial individual differences in their levels of physical activity were unlikely.
Phenotype indicators
Body composition
Body density was measured by the underwater weighing technique 17, and the percentage of body fat (%fat) was calculated with the equation of Siri 18. The cross-sectional areas of abdominal subcutaneous and visceral adipose tissues were measured by computerized tomography as previously described 19.
Plasma lipoprotein concentrations
Blood samples were collected from an antecubital vein in vacutainer tubes containing EDTA. Samples were taken in the morning after a 12-hour fast. Plasma lipoprotein fractions were prepared by the combined use of ultracentrifugation 20 and heparin-manganese precipitation 21. Details of these assays were described in an earlier publication 22.
Genotype markers
Genomic DNA extracted from lymphoblastoid cell lines by conventional phenol-chloroform methods was used in PCR amplifications 23. Briefly, the PCR reactions were carried with genomic DNA (200 ng), and the PCR product was digested with the appropriate restriction enzyme. Five regions of the CETP gene were amplified in separate PCR reactions and digested with restriction enzymes (ie, TaqIB, intron 1; MspI, intron 8; BamHI, intron 9; TaqIA, intron 10; and RsaI, exon 14) as suggested by Kuivenhoven 24. The oligonucleotide sets used with TaqI (A and B) and MspI were described by Drayna and Lawn 25 and the primer pairs used with BamHI and RsaI were described by Kuivenhoven 24. Following the recommendations of the Nomenclature Working Group for gene names, symbols, and polymorphism descriptions (http://archive.uwcm.ac.uk/uwcm/mg/docs//mut_nom.html), the GenBank sequence AC010550.7 was used as the numbering reference (start of transcription as position +1). CETP BamHI RFLP corresponds to position +11,592 and is referred to as C>T/In9 (rs289714) herein. CETP RsaI RFLP corresponds to position +20,233 and is designated as G>A/Ex14 (rs5882); this variant confers a non-synonymous amino acid change of isoleucine (I) to valine (V), and is commonly known as I405V 26. According to NCBI RefSeq NM_000078.1. the amino acid change is located at position 422, and not at 405, as it has been previously reported 27.
Statistical analysis
Differences between genotypes in the phenotypic response to overfeeding were analyzed by t-tests. Percentage changes were calculated from individual scores. Analyses were performed both with the 24 subjects considered as unrelated persons and with the phenotype means of each of the 12 pairs. The SAS statistical package (SAS Institute Inc., Cary, NC) was used to perform all statistical analyses.
RESULTS
Changes in body composition and lipoprotein profile in the overfeeding study have been reported previously 22. Here, we report on changes related to two CETP gene single nucleotide polymorphisms (SNPs), C>T/In9 and G>A/Ex14, which are not in linkage disequilibrium. We detected no differences among the pairs of twins for the introns 1, 8 and 10 polymorphisms.
C>T/In9 CETP polymorphism
Eighteen subjects were CC homozygotes at the C>T/In9 variant and six were CT heterozygotes. Table 1 and Table 2 depict changes in body composition and lipoprotein profile by genotype. CT heterozygotes gained more weight with the overfeeding protocol than CC homozygotes (Table 1). Individuals with the CT genotype had higher %fat and fat mass (FM) than the CC homozygotes at baseline. These differences were constant; CT heterozygotes gained significantly more weight and FM. After overfeeding, HDL3 levels were 19% lower in CT subjects (P = 0.05). No other significant differences were observed between the two genotypes.
Table 1.
Effect of 100 days of overfeeding on total and abdominal fatness in relation to the CETP C>T/In9 genotype.
| Variables | CC/In9, n=18 Mean (SE) * | CT/In9, n=6 Mean (SE) | P value |
|---|---|---|---|
| Weight (kg) | |||
| Before | 58.8 (2.9) | 64.9 (1.7) | NS |
| Change † | 7.3 (0.4) | 10.6 (1.5) | 0.01 |
| BMI (kg/m2) | |||
| Before | 19.5 (0.8) | 20.2 (0.6) | NS |
| Change | 2.5 (0.2) | 3.3 (0.4) | 0.04 |
| Body Fat (%) | |||
| Before | 10.0 (1.6) | 15.1 (2.1) | 0.02 |
| Change | 6.2 (0.8) | 7.6 (0.6) | NS |
| Fat mass (kg) | |||
| Before | 6.0 (1.1) | 9.7 (1.2) | 0.01 |
| Change | 4.8 (0.4) | 7.3 (0.7) | 0.01 |
| Fat-free mass (kg) | |||
| Before | 52.8 (2.4) | 55.2 (2.6) | NS |
| Change | 2.6 (0.4) | 3.2 (0.9) | NS |
| CT derived adipose tissue areas | |||
| Subcutaneous (cm2) | |||
| Before | 60.6 (8.0) | 106.9 (36.6) | NS |
| Change | 64.4 (3.4) | 80.4 (8.8) | 0.05 |
| Visceral (cm2) | |||
| Before | 32.3 (3.2) | 39.1 (1.7) | NS |
| Change | 24.7 (3.9) | 23.3 (7.3) | NS |
Values are means (SE), using the average value of each twin pair.
Change: Post-overfeeding minus pre-overfeeding values.
NS: No significant differences between genotypes were observed.
Table 2.
Effect of 100 days of overfeeding on plasma lipoproteins levels in relation to the CETP C>T/In9 genotype.
| Variable | CC/In9, n=18 Mean (SE) * | CT/In9, n=6 Mean (SE) |
|---|---|---|
| Cholesterol (mmol/l) | ||
| Before | 4.4 (0.3) | 4.6 (0.4) |
| Change† | 0.4 (0.3) | 0.5 (0.4) |
| Triglycerides (mmol/l) | ||
| Before | 1.2 (0.2) | 1.2 (0.1) |
| Change | 0.5 (0.3) | 0.9 (0.4) |
| VLDL-C (mmol/l) | ||
| Before | 0.4 (0.1) | 0.5 (0.1) |
| Change | 0.2 (0.2) | 0.2 (0.2) |
| LDL-C (mmol/l) | ||
| Before | 2.8 (0.2) | 3.1 (0.4) |
| Change | 0.3 (0.2) | 0.4 (0.3) |
| LDL-apo B (mg/dl) | ||
| Before | 67.7 (5.2) | 77.2 (8.5) |
| Change | 7.4 (4.5) | 10.7 (8.8) |
| HDL-C (mmol/l) | ||
| Before | 1.2 (0.1) | 1.1 (0.1) |
| Change | −0.1 (0.0) | −0.1 (0.1) |
| HDL-apo A-I (mg/dl) | ||
| Before | 112.2 (5.7) | 105.3 (3.2) |
| Change | −4.3 (4.7) | −3.0 (4.2) |
| HDL2 (mmol/l) | ||
| Before | 0.50 (0.03) | 0.40 (0.01) |
| Change | −0.03 (0.03) | −0.03 (0.1) |
| HDL3 (mmol/l) | ||
| Before | 0.75 (0.03) | 0.67 (0.12) |
| Change | −0.05 (0.02) | −0.10 (0.09) |
Values are means (SE), using the mean value of each twin pair. No significant differences between genotypes were observed.
Change: Post-overfeeding minus pre-overfeeding values.
G>A/Ex14 polymorphism (I405V)
Table 3 presents the body composition characteristics by genotype and Table 4 the lipoprotein profile. Eight subjects were V405V homozygous, ten heterozygous, and six I405I homozygous. The V405V homozygotes had lower BMI, %fat, FM, and abdominal subcutaneous fat (ASF) areas before overfeeding than 405I carriers (Table 3). Despite their lower adiposity, the V405V homozygotes gained more AVF with overfeeding (P = 0.02). Considering the change with overfeeding as a percent increase from baseline, there was a trend for more gain in fat mass (148% vs. 67%, P = 0.06) and AVF (130% vs. 52%, P = 0.09) in V405V homozygotes compared to I405I homozygotes.
Table 3.
Effect of 100 days of overfeeding on total and abdominal fatness in relation to the I405V CETP genotype.
| Variables | V405V, n=8 Mean (SE) * | I405V, n=10 Mean (SE) | I405I, n=6 Mean (SE) | P value |
|---|---|---|---|---|
| BMI (kg/m2) | ||||
| Before | 17.6 (0.7) | 20.8 (0.6) | 20.6 (0.8) | 0.02 |
| Change† | 2.7 (0.3) | 3.0 (0.3) | 2.2 (0.3) | NS |
| Body Fat (%) | ||||
| Before | 7.9 (2.3) | 13.5 (2.1) | 12.0 (2.7) | 0.05 |
| Change | 7.3 (1.1) | 6.6 (1.0) | 5.3 (1.2) | NS |
| Fat mass (kg) | ||||
| Before | 4.0 (1.4) | 8.7 (1.3) | 7.8 (1.6) | 0.007 |
| Change | 4.9 (0.8) | 6.2 (0.7) | 4.6 (1.0) | NS |
| Fat-free mass (kg) | ||||
| Before | 47.3 (2.6) | 56.5 (2.3) | 56.1 (3.0) | 0.0002 |
| Change | 2.8 (1.0) | 2.9 (1.3) | 2.3 (1.1) | NS |
| Sum 8 skin folds (mm) | ||||
| Before | 67.1 (7.6) | 97.5 (6.8) | 85.5 (8.8) | 0.02 |
| Change | 56.5 (8.4) | 85.9 (7.5) | 70.8 (9.7) | NS |
| CT derived adipose tissue areas | ||||
| Subcutaneous (cm2) | ||||
| Before | 46.7 (18.1) | 96.7 (16.1) | 65.5 (20.9) | 0.02 |
| Change | 65.4 (6.2) | 75.3 (5.6) | 60.8 (7.2) | NS |
| Visceral (cm2) | ||||
| Before | 29.4 (4.5) | 37.9 (4.0) | 33.6 (5.1) | NS |
| Change | 33.9 (4.7) | 21.3 (4.2) | 16.6 (5.4) | 0.02 |
Values are means (SE), using the average value of each twin pair
Change: Post-overfeeding minus pre-overfeeding values.
NS: No significant differences between genotypes were observed.
Table 4.
Effect of 100 days of overfeeding on lipoprotein fractions in relation to the I405V CETP genotype.
| Variable | V405V, n=8 Mean (SE) * | I405V, n=10 Mean (SE) | I405I, n=6 Mean (SE) | P value |
|---|---|---|---|---|
| Cholesterol (mmol/l) | ||||
| Before | 4.5 (0.4) | 4.9 (0.3) | 3.9 (0.3) | NS |
| Change † | 0.8 (0.7) | 0.2 (0.3) | 0.3 (0.1) | NS |
| Triglycerides (mmol/l) | ||||
| Before | 1.22 (0.40) | 1.21 (0.08) | 0.97 (0.26) | NS |
| Change † | 0.86 (0.76) | 0.69 (0.26) | 0.26 (0.24) | NS |
| VLDL-C (mmol/l) | ||||
| Before | 0.44 (0.18) | 0.47 (0.06) | 0.34 (0.08) | NS |
| Change † | 0.39 (0.38) | 0.16 (0.10) | 0.11 (0.11) | NS |
| VLDL-apo B (mg/dl) | ||||
| Before | 6.5 (1.7) | 7.7 (1.8) | 5.3 (0.7) | NS |
| Change † | 6.6 (4.1) | 4.4 (1.6) | 1.7 (0.9) | NS |
| LDL-C (mmol/l) | ||||
| Before | 2.7 (0.3) | 3.3 (0.3) | 2.4 (0.3) | 0.02 |
| Change † | 0.4 (0.3) | 0.2 (0.2) | 0.3 (0.1) | NS |
| ApoB (mg/dl) | ||||
| Before | 69.5 (6.9) | 90.1 (7.2) | 64.3 (5.6) | 0.004 |
| Change † | 19.4 (13.3) | 12.0 (7.0) | 4.8 (3.0) | NS |
| HDL-C (mmol/l) | ||||
| Before | 1.31 (0.08) | 1.08 (0.09) | 1.17 (0.01) | 0.02 |
| Change † | −0.04 (0.08) | −0.12 (0.05) | −0.11 (0.02) | 0.01 |
| Adjusted change ‡ | 0.02 (0.04) | −0.16 (0.03) | −0.12 (0.04) | 0.02 |
| HDL2 (mmol/l) | ||||
| Before | 0.51 (0.06) | 0.40 (0.03) | 0.45 (0.03) | NS |
| Change † | 0.01 (0.06) | −0.02 (0.03) | −0.07 (0.03) | 0.05 |
| Adjusted change ‡ | 0.03 (0.03) | −0.04 (0.02) | −0.08 (0.03) | 0.04 |
| HDL3 (mmol/l) | ||||
| Before | 0.80 (0.02) | 0.68 (0.08) | 0.73 (0.03) | NS |
| Change † | −0.04 (0.02) | −0.10 (0.05) | −0.03 (0.02) | 0.0004 |
| Adjusted change ‡ | −0.004 (0.02) | −0.13 (0.02) | −0.04 (0.02) | 0.002 |
| HDL-apo A-I (mg/dl) | ||||
| Before | 123.8 (10.0) | 103.2 (3.2) | 105.0 (1.3) | 0.005 |
| Change † | −4.6 (11.4) | −3.7 (2.5) | −3.7 (2.4) | 0.05 |
| Adjusted change ‡ | 2.7 (5.5) | −7.0 (4.4) | −7.9 (5.2) | NS |
Values are means (SE), using the mean value of each twin pair
NS: No significant differences between genotypes were observed.
Change: Post-overfeeding minus pre-overfeeding values.
Adjusted change: Post-overfeeding minus pre-overfeeding values adjusted for baseline, baseline VLDL-TG and VLDL-TG change with overfeeding.
The I405V genotypes differed also in the lipoprotein profile (Table 4). Before overfeeding, V405V homozygotes had higher levels of HDL-C, and HDL-Apo AI. With overfeeding, decreases from baseline were significantly different among the three genotypes for HDL-C (P = 0.01), HDL-Apo AI (P = 0.05), HDL2 (P = 0.05), and HDL3 (P = 0.0004) levels.
In response to overfeeding, VLDL-TG levels increased in all subjects. VLDL-TG concentration increased from 0.70 ± 0.13 to 1.22 ± 0.34 mmol/l after overfeeding. Given the VLDL-TG increase with overfeeding in all subjects, possibly affecting HDL-CE transfer to LDL or VLDL particles 9, we investigated the CETP I405V genotypes in this response. When HDL subfractions were adjusted statistically for both VLDL-TG at baseline and VLDL-TG changes with overfeeding, there were significant differences in response to overfeeding for HDL-C, HDL2, and HDL3 levels among the I405V genotypes (Table 4). After adding VLDL-TG as a covariate in the analyses of HDL-C subfractions post-overfeeding, higher concentrations of HDL-C, HDL2 and HDL3 were observed in V405V subjects compared to I405I homozygotes. In response to overfeeding, I405I homozygotes consistently decreased their HDL-C (P = 0.02), HDL2 (P = 0.04) and HDL3 (P = 0.002) concentrations compared to the V405V homozygotes (Table 4), whether subjects were considered as 12 pairs or as 24 individuals.
DISCUSSION
CETP has both pro- and anti-atherogenic effects 9, 10, 28. It is apparently anti-atherogenic with high HDL levels6 but negative correlations between CETP and HDL-C or the ratio of TG/CE in HDL2 and HDL3 particles in obese individuals suggest atherogenicity 29.
Studies on the relationship between CETP polymorphisms and CHD risk levels are inconclusive. Cross-sectional studies of the CETP I405V polymorphism have shown either no association with the risk of CHD or lipid parameters 30 or higher HDL levels and increased risk of CHD for male V-allele homozygotes2. No data exist on the effects of CETP polymorphisms on the adaptation to chronic overfeeding but a few studies have positively correlated circulating CETP levels and obesity 28, 29, 31.
Heterozygotes for the C>T/In9 polymorphism had more ASF before overfeeding and gained 25% more ASF with overfeeding compared to the CC/In9 homozygotes, even with BMI never exceeding 25 kg/m2. This could have clinical implications, as the regional distribution of adipose tissue is considered an independent risk factor for metabolic diseases.
Interestingly, individuals with the CETP V405V genotype had the lowest ASF before overfeeding, but the highest percentage increase in AVF with overfeeding (130%). Despite this, the V405V homozygotes had higher HDL-C, HDL-Apo AI, HDL2, and HDL3 levels. Higher CETP mRNA levels in adipocytes of subcutaneous depots, compared to those of the visceral depot, have been observed in obese individuals 32. The 405V allele has been consistently related to decreased CETP mass and activity 10. We speculate that the increased adipose tissue mass might contribute to higher levels of CETP mRNA possibly with higher circulating CETP protein levels compensating for the lower activity of CETP associated with the 405V allele.
This overfeeding intervention was in young lean males with normal baseline lipid profiles. Hence, the observed gene-overfeeding interactions could be different and perhaps stronger in older or in high-risk individuals. The deleterious effects of the CETP V405V and CT/In9 genotypes may be more apparent in subjects with higher BMI or other risk factors. One could speculate that phenotypic associations seen here may represent only early stages of more complex metabolic anomalies.
In conclusion, DNA sequence variants in the CETP gene are associated with inter-individual differences observed in the response of HDL-C and adiposity to long-term positive energy balance. Although the sample size was small and the subjects young and healthy, these data suggest that CETP sequence variants may help to recognize individuals who are at higher risk of cardiovascular disease or metabolic syndrome.
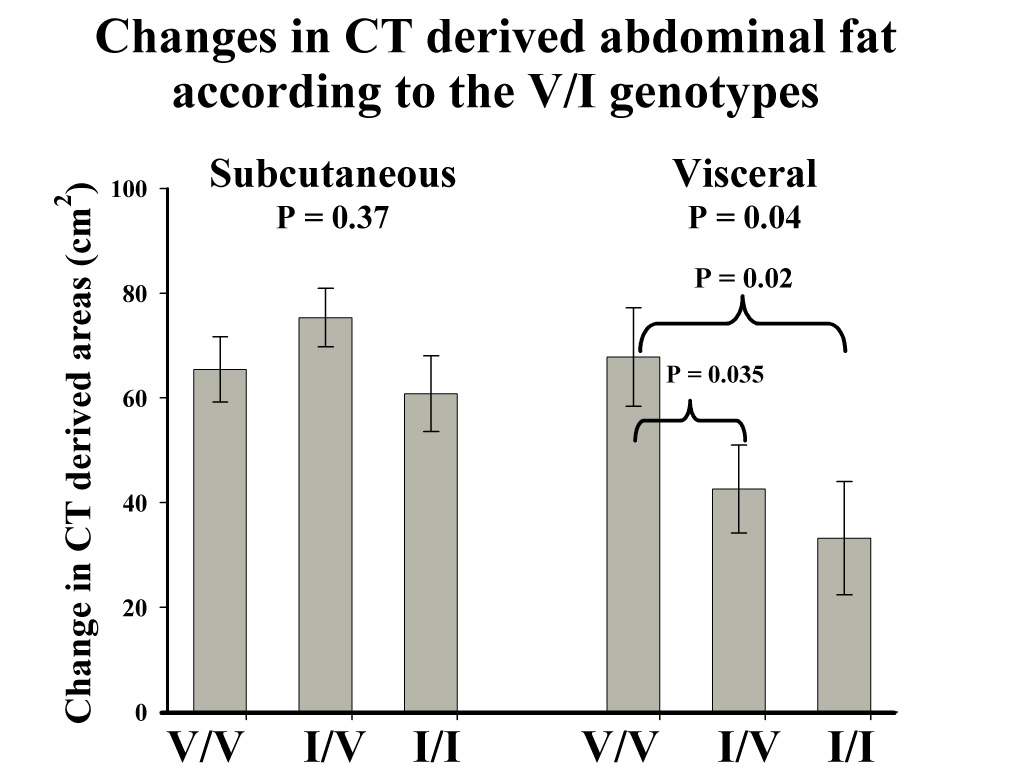
Changes in CT derived abdominal fat by the I405V CETP genotype in response to 100 days of overfeeding. Bars represent the mean ± SE. Data analyzed as the mean change in each of the 12 pairs of twins.
ACKNOWLEDGEMENTS
We are indebted to Jacques Bouillon, Suzie Hamel, Brigitte Zément, Maryse Lebrun, Martine Marcotte, Monique Chagnon, Josée Lapointe, Henri Bessette, Gilles Bouchard and Serge Carbonneau for their contributions to this study. Special thanks go to Guy Fournier and Dr Germain Thériault for their role in the management of the study and to Claude Leblanc for his statistical support. Thanks to Nina Laidlaw for her editorial support. Supported in part by a grant (DK 34624) from the National Institutes of Health. C. Bouchard is supported in part by the George A Bray Chair in Nutrition.
This work was supported by a grant from the National Institutes of Health DK34624.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.McGill HC, Jr, McMahan CA, Herderick EE, et al. Obesity Accelerates the Progression of Coronary Atherosclerosis in Young Men. Circulation. 2002;105:2712–2718. doi: 10.1161/01.cir.0000018121.67607.ce. [DOI] [PubMed] [Google Scholar]
- 2.Bruce C, Chouinard RA, Jr, Tall AR. Plasma lipid transfer proteins, high density lipoproteins, and reverse cholesterol transport. Annu Rev Nutr. 1998;18:297–330. doi: 10.1146/annurev.nutr.18.1.297. [DOI] [PubMed] [Google Scholar]
- 3.Bruce C, Davidson WS, Kussie P, et al. Molecular determinants of plasma cholesteryl ester transfer protein binding to high density lipoproteins. J Biol Chem. 1995;270:11532–11542. doi: 10.1074/jbc.270.19.11532. [DOI] [PubMed] [Google Scholar]
- 4.Quinet EM, Agellon LB, Kroon PA, et al. Atherogenic diet increases cholesteryl ester transfer protein messenger RNA levels in rabbit liver. J Clin Invest. 1990;85:357–363. doi: 10.1172/JCI114446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fusegawa Y, Kelley KL, Sawyer JK, et al. Influence of dietary fatty acid composition on the relationship between CETP activity and plasma lipoproteins in monkeys. J Lipid Res. 2001;42:1849–1857. [PubMed] [Google Scholar]
- 6.Ordovas JM, Cupples LA, Corella D, et al. Association of cholesteryl ester transfer protein-TaqIB polymorphism with variations in lipoprotein subclasses and coronary heart disease risk: the Framingham study. Arterioscler Thromb Vasc Biol. 2000;20:1323–1329. doi: 10.1161/01.atv.20.5.1323. [DOI] [PubMed] [Google Scholar]
- 7.Zhong S, Sharp DS, Grove JS, et al. Increased coronary heart disease in Japanese-American men with mutation in the cholesteryl ester transfer protein gene despite increased HDL levels. J Clin Invest. 1996;97:2917–2923. doi: 10.1172/JCI118751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barter P. CETP and Atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20:2029–2031. doi: 10.1161/01.atv.20.9.2029. [DOI] [PubMed] [Google Scholar]
- 9.Barter PJ, Brewer HB, Jr, Chapman MJ, et al. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:160–167. doi: 10.1161/01.atv.0000054658.91146.64. [DOI] [PubMed] [Google Scholar]
- 10.Boekholdt SM, Thompson JF. Natural genetic variation as a tool in understanding the role of CETP in lipid levels and disease. J Lipid Res. 2003;44:1080–1093. doi: 10.1194/jlr.R200018-JLR200. [DOI] [PubMed] [Google Scholar]
- 11.Tall A. Plasma lipid transfer proteins. Annu Rev Biochem. 1995;64:235–257. doi: 10.1146/annurev.bi.64.070195.001315. [DOI] [PubMed] [Google Scholar]
- 12.Medina WL, Nunes VS, Carrilho AJ, et al. High-density lipoprotein cholesterol esterification and transfer rates to lighter density lipoproteins mediated by cholesteryl ester transfer protein in the fasting and postprandial periods are not altered in type 1 diabetes mellitus. Eur J Intern Med. 2000;11:264–270. doi: 10.1016/s0953-6205(00)00101-1. [DOI] [PubMed] [Google Scholar]
- 13.Lottenberg AM, Nunes VS, Nakandakare ER, et al. The human cholesteryl ester transfer protein I405V polymorphism is associated with plasma cholesterol concentration and its reduction by dietary phytosterol esters. J Nutr. 2003;133:1800–1805. doi: 10.1093/jn/133.6.1800. [DOI] [PubMed] [Google Scholar]
- 14.Wilund KR, Ferrell RE, Phares DA, et al. Changes in high-density lipoprotein-cholesterol subfractions with exercise training may be dependent on cholesteryl ester transfer protein (CETP) genotype. Metabolism. 2002;51:774–778. doi: 10.1053/meta.2002.32730. [DOI] [PubMed] [Google Scholar]
- 15.Bouchard C, Tremblay A, Despres JP, et al. The response to long-term overfeeding in identical twins. N Engl J Med. 1990;322:1477–1482. doi: 10.1056/NEJM199005243222101. [DOI] [PubMed] [Google Scholar]
- 16.Verdier P, Beare-Rogers JL. The Canadian Nutrient File. J Can Diet Assoc. 1984;45:52–55. [PubMed] [Google Scholar]
- 17.Behnke AR, Wilmore JH. Evaluation and regulation of body build and composition. Englewood Cliffs NJ: Prentice-Hall; 1974. p. 236 pages. [Google Scholar]
- 18.Siri WE. The gross composition of the body. Adv Biol Med Phys. 1956;4:239–290. doi: 10.1016/b978-1-4832-3110-5.50011-x. [DOI] [PubMed] [Google Scholar]
- 19.Ferland M, Despres JP, Tremblay A, et al. Assessment of adipose tissue distribution by computed axial tomography in obese women: association with body density and anthropometric measurements. Br J Nutr. 1989;61:139–148. doi: 10.1079/bjn19890104. [DOI] [PubMed] [Google Scholar]
- 20.Havel RJ, Eder H, Bragdon HF. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brustein M, Samaille J. Sur un dosage rapide du cholestérol lié aux B-lipoprotéines du sérum. Clin Chim Acta. 1960;5:609–610. [Google Scholar]
- 22.Bouchard C, Tremblay A, Despres JP, et al. Overfeeding in identical twins: 5-year postoverfeeding results. Metabolism. 1996;45:1042–1050. doi: 10.1016/s0026-0495(96)90277-2. [DOI] [PubMed] [Google Scholar]
- 23.Neitzel H. A routine method for the establishment of permanent growing lymphoblastoid cell lines. Hum Genet. 1986;73:320–326. doi: 10.1007/BF00279094. [DOI] [PubMed] [Google Scholar]
- 24.Kuivenhoven JA, de Knijff P, Boer JM, et al. Heterogeneity at the CETP gene locus. Influence on plasma CETP concentrations and HDL cholesterol levels. Arterioscler Thromb Vasc Biol. 1997;17:560–568. doi: 10.1161/01.atv.17.3.560. [DOI] [PubMed] [Google Scholar]
- 25.Drayna D, Lawn R. Multiple RFLPs at the human cholesteryl ester transfer protein (CETP) locus. Nucleic Acids Res. 1987;15:4698. doi: 10.1093/nar/15.11.4698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson JF, Lira ME, Durham LK, et al. Polymorphisms in the CETP gene and association with CETP mass and HDL levels. Atherosclerosis. 2003;167:195–204. doi: 10.1016/s0021-9150(03)00005-4. [DOI] [PubMed] [Google Scholar]
- 27.Agellon LB, Quinet EM, Gillette TG, et al. Organization of the human cholesteryl ester transfer protein gene. Biochemistry. 1990;29:1372–1376. doi: 10.1021/bi00458a004. [DOI] [PubMed] [Google Scholar]
- 28.Asayama K, Hayashibe H, Dobashi K, et al. Increased serum cholesteryl ester transfer protein in obese children. Obes Res. 2002;10:439–446. doi: 10.1038/oby.2002.61. [DOI] [PubMed] [Google Scholar]
- 29.Arai T, Yamashita S, Hirano K, et al. Increased plasma cholesteryl ester transfer protein in obese subjects. A possible mechanism for the reduction of serum HDL cholesterol levels in obesity. Arterioscler Thromb. 1994;14:1129–1136. doi: 10.1161/01.atv.14.7.1129. [DOI] [PubMed] [Google Scholar]
- 30.Pallaud C, Gueguen R, Sass C, et al. Genetic influences on lipid metabolism trait variability within the Stanislas Cohort. J Lipid Res. 2001;42:1879–1890. [PubMed] [Google Scholar]
- 31.Dullaart RP, Sluiter WJ, Dikkeschei LD, et al. Effect of adiposity on plasma lipid transfer protein activities: a possible link between insulin resistance and high density lipoprotein metabolism. Eur J Clin Invest. 1994;24:188–194. doi: 10.1111/j.1365-2362.1994.tb00987.x. [DOI] [PubMed] [Google Scholar]
- 32.Dusserre E, Moulin P, Vidal H. Differences in mRNA expression of the proteins secreted by the adipocytes in human subcutaneous and visceral adipose tissues. Biochim Biophys Acta. 2000;1500:88–96. doi: 10.1016/s0925-4439(99)00091-5. [DOI] [PubMed] [Google Scholar]


