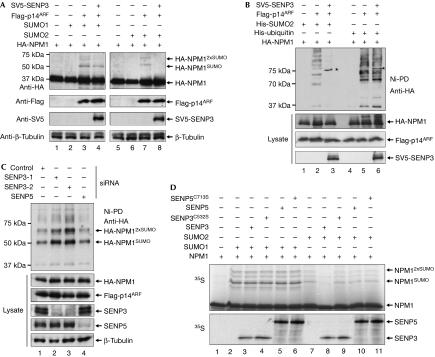
Figure 2.
SENP3 catalyses the desumoylation of nucleophosmin. (A) HA-NPM1 was expressed alone or together with Flag-p14ARF and SUMO1 or SUMO2 in the presence or absence of SV5-tagged SENP3 in human embryonic kidney (HEK)293T cells. Expression of the respective proteins was verified by immunoblotting. Western blotting with anti-tubulin shows equal loading. (B) HA-NPM1 and His-tagged versions of SUMO2 or ubiquitin were expressed in the presence of Flag-p14ARF and SV5-SENP3 in HEK293T cells. His–SUMO2 or His–ubiquitin conjugates were recovered on Ni-NTA beads and subjected to western blotting using an HA antibody. Expression of the respective proteins was monitored by western blotting of cell lysates. Asterisks indicate a crossreactivity of the HA antibody with overexpressed SV5-tagged SENP3. (C) HEK293T cells were depleted from SENP3 or SENP5 by siRNA duplexes and transfected with the indicated plasmids. His–SUMO2 conjugates were recovered on Ni-NTA beads and subjected to western blotting using an HA antibody. Expression of the respective proteins was monitored by western blotting of cell lysates. (D) 35S-labelled NPM1, generated by in vitro transcription/translation, was first incubated with recombinant E1 enzyme, E2 enzyme and either SUMO1 (lanes 2–6) or SUMO2 (lanes 7–11) in the presence of ATP. In the control reaction, SUMO was omitted (lane 1). Following the modification reaction, SENP3 (lanes 3,8) and SENP5 (lanes 5,10) or the catalytically inactive mutants SENP3C532S (lanes 4,9) and SENP5C713S (lanes 6,11), generated by in vitro translation/transcription, were added. The amounts of SENP3, SENP5 and the respective catalytically inactive mutants are shown below. HA, haemagglutinin; NPM1, nucleophosmin; siRNA, short interfering RNA.