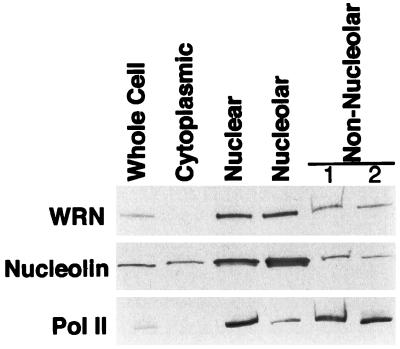
Figure 4.
Distribution of WRN protein among HeLa subcellular fractions. Extracts of HeLa cells were prepared and fractionated into cytoplasmic, nuclear, nucleolar, and nonnucleolar fractions. A 10-μg sample of each fraction was separated by electrophoresis on an SDS/4–15% polyacrylamide gradient gel and then analyzed by Western blotting. Blots, prepared in parallel, were probed with the antibodies indicated to the left. To control for the fidelity of the fractionation, blots were probed with antibodies to proteins of preestablished subnuclear localization: nucleolin, which is present in increased concentration in the nucleolus, and RNA polymerase II (Pol II), which is excluded from the nucleolus. Nonnucleolar lanes 1 and 2 are the nonsedimented material from the first and second cycle of sedimentation through sucrose (see Materials and Methods). An obvious decrease in concentration of WRN or nucleolin in the total nuclear compared with the nucleolar fractions was not observed. There are two possible explanations for this finding. The nucleolar fraction is contaminated with some amount of adherent chromatin, and some nucleolar proteins diffuse out of nucleoli during fractionation. Both of these artifacts will decrease the apparent concentration of a protein in the nucleolar fraction. The diffusion of nucleolar protein during fractionation will have a lesser effect on its concentration in the nonnucleolar fraction, as the nucleolar fraction contains 20% of the total nuclear protein, whereas the nonnucleolar fraction contains 80%.