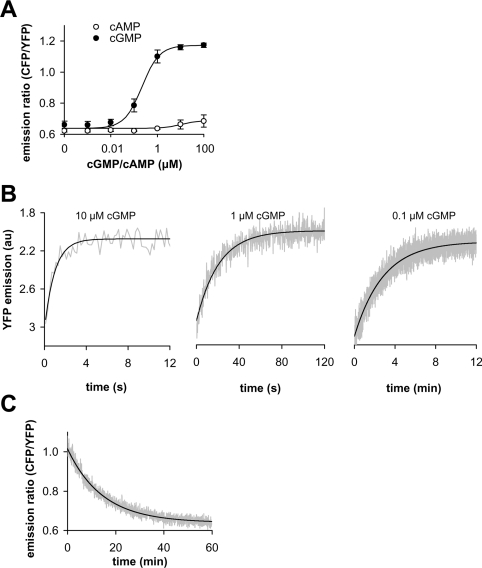
Figure 2. Ligand-binding characteristics of a cGMP-sensitive construct designed from the tandem domains of PDE5 GAF A+B.
(A) Concentration–response curves of PDE5-GAF A+B (N-terminally elongated by 51 amino acids and C-terminally shortened by two amino acids) for cGMP and cAMP were determined in vitro from the change in emission ratio at 475 nm (CFP emission) to 525 nm (YFP emission). (B) Binding kinetics at 0.1–10 μM cGMP were recorded using a rapid-mixing device at 525 nm (YFP) (note the different time scales). Original trace data (grey lines) were fitted to single exponential kinetics (black lines) and yielded half-lives of approx. 0.7, 14, and 120 s at 10, 1 and 0.1 μM cGMP respectively. (C) Dissociation of cGMP was assessed as a change in the emission ratio after a 100-fold dilution following binding of 1 μM cGMP. Original trace data (grey) were fitted to single exponential kinetics (black); the half-life was approx. 10 min.