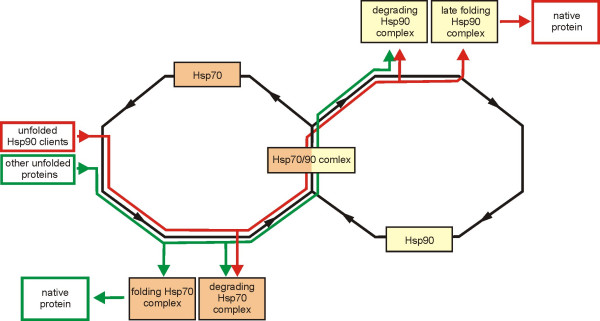
Figure 3.
Substrate selection and processing in the eukaryotic Hsp70/Hsp90 chaperone system. Newly synthesized or secondarily unfolded proteins are recognized and bound by Hsp70. These can be either proteins that require assistance of Hsp90 at later steps of their folding or any other polypeptides. The latter (green line) can attain their native configuration with the assistance of the folding Hsp70 complex [18]. or be disposed with the help of the degrading Hsp70 complex if they are excessively unstable [34]. It is also possible that proteins that normally do not need to interact with Hsp90 in the process of their folding are nevertheless passed to the degrading Hsp90 complex when they have to be disposed [79]. Proteins that do require Hsp90 for the late steps of their folding (red line) can be directed to degradation before contacting this chaperone if they are excessively unstable [31]. Otherwise, they are passed from Hsp70 to Hsp90 through an intermediate complex containing both these chaperones. Maturation of these proteins can be finished in the late folding Hsp90 complex [82] or aborted in the degrading Hsp90 complex if they do not follow expected scenario of folding [84]. Molecules which need to be assisted by Hsp90 but are dropped before their fold is completed have to re-enter their path of chaperone-assisted maturation primarily, or perhaps exclusively through binding to the Hsp70 molecules [83]. This renders them to another round of quality control.