Fig. 4.
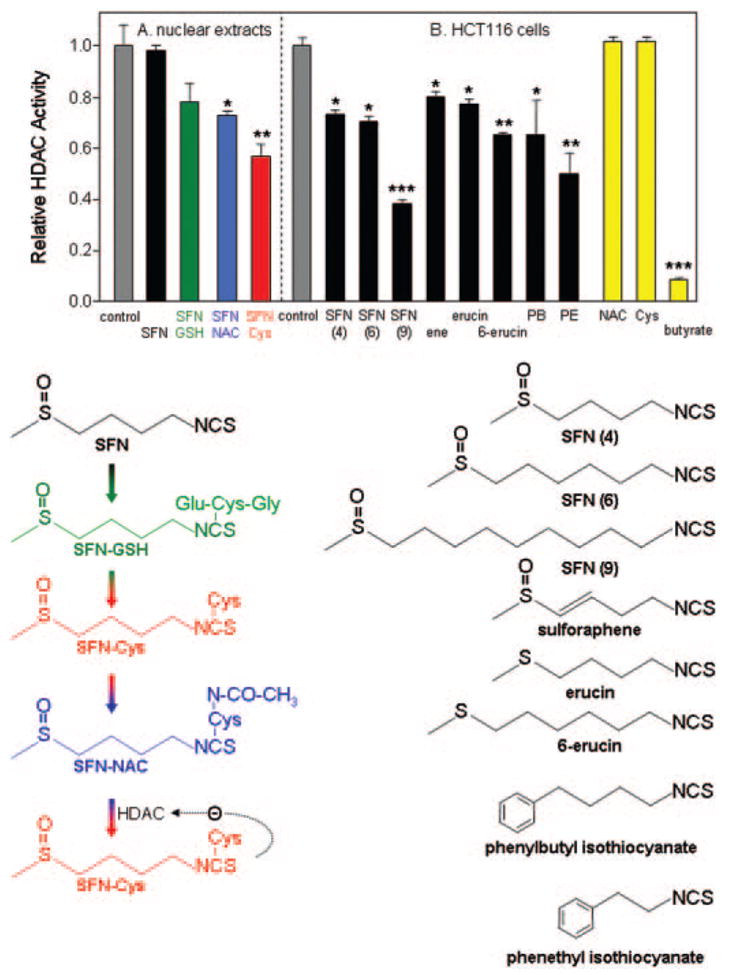
HDAC inhibitory activity of sulforaphane (SFN) and structurally related isothiocyanates. (A) The mercapturic acid pathway converts SFN sequentially to SFN–GSH (SFN–glutathione), SFN–Cys (SFN–cysteine) and SFN–NAC (SFN–N-acetylcysteine). SFN–Cys formed from SFN–GSH, or after deacetylation of SFN–NAC by HDAC, leads to competitive enzyme inhibition, according to the working hypothesis (see text). Direct addition of SFN or SFN–GSH to isolated nuclear extracts in vitro had no significant effect on HDAC activity, whereas SFN–NAC (blue bar) and SFN–Cys (red bar) attenuated HDAC activity significantly. (B) Incubation of SFN with HCT116 human colon cancer cells followed by testing of the cell lysates revealed significant HDAC inhibition, as seen with several other structurally related isothiocyanates. All concentrations were 15 μM, except butyrate which was 1 mM. Data = mean ± SD, n =3. *P < 0.05, **P <0.01, ***P < 0.001, using Student’s t-test versus the corresponding control (gray bar). For details of the HDAC assay and other conditions, see (30).
