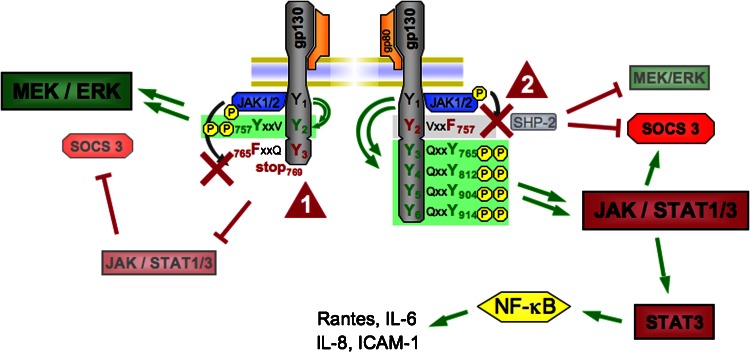
Figure 2.
Mutations in the glycoprotein 130 (gp130) receptor lead to an imbalance in downstream signalling pathways. Detailed schematic view of the gp130 receptor structure shows tyrosine 757 (Y757) necessary for SH2 domain-containing cytoplasmic protein tyrosine phosphatase (SHP2) phosphorylation and suppressor of cytokine signalling (SOCS)-3 binding. A point mutation (Y757 → F757, tyrosine → phenylalanine) abolishes binding of SHP2 and SOCS3 (arrowhead 1) and leads to uncontrolled Janus kinase (JAK)/signal transducer and activator of transcription (STAT) activation with exaggerated STAT3 activation. As a consequence, proinflammatory pathways, that is nuclear factor-κB (NF-κB) activation, are promoted. The left side displays a truncated gp130 intracellular domain incapable of binding and activating the STAT1/3 pathway (arrowhead 2) from its membrane-distal phosphotyrosine residues. This mutant form is characterized by the substitution of Y765 by F765 and is truncated at the C-terminal side of amino acid 768. Deficient STAT1/3 signalling in this model is accompanied by enhanced signalling via the SHP2/MEK/extracellular signal-regulated kinase (ERK) pathway. ICAM, intercellular adhesion molecule-1. MEK, mitogen-activated protein kinase (MAPK)/ERK kinase.