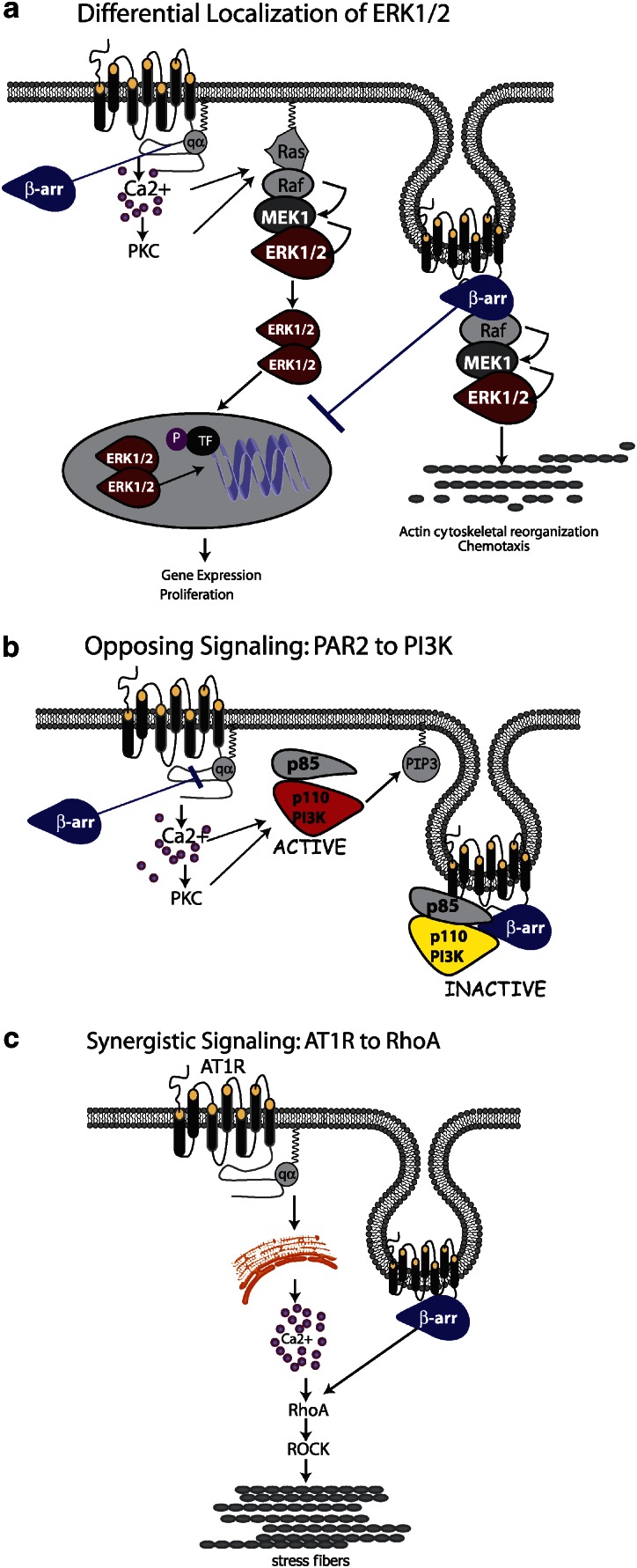
Figure 1.
Types of β-arrestin-dependent, G-protein-independent signals. (a) Differential localization of ERK1/2. β-Arrestins can activate ERK1/2 downstream of numerous GPCRs and effectively ‘steal' the kinases away the G-protein-dependent pathway. In the case of PAR-2 and AT1R, signalling through Gαq leads to mobilization of intracellular Ca2+, activation of conventional PKCs and Ras-dependent activation of the MAPK module (Raf-1, MEK1/2 and ERK1/2). The activated ERKs translocate to the nucleus where they phosphorylate transcription factors (TF) leading to gene expression and proliferation. When activated through β-arrestins, the entire MAPK module is scaffolded onto the β-arrestin-bound receptor, forming an ‘endosomal scaffold' that promotes prolonged activation of ERK1/2 at the membrane or within the cytosol preventing the transcriptional and proliferative effects and promoting cytoskeletal reorganization and chemotaxis. (b) Opposing signalling: PAR-2 to PI3K. PAR-2 can both activate and inhibit PI3K in a cell-type-specific fashion, depending on the expression level of β-arrestins. Activation of the classical Gaq/Ca2+ pathway leads to increased activity of the p110 catalytic subunit of PI3K, leading to generation of PIP3. Recruitment of β-arrestins leads to the formation of a unique endosomal scaffold containing the regulatory (p85) and catalytic PI3K subunits. Binding to β-arrestins directly inhibits PI3K activity. (c) Synergistic signalling: AT1R and RhoA. AT1R activates RhoA, leading to stress fibre formation, through activation of the RhoA effector ROCK. Both β-arrestin-dependent recruitment to the receptor and coupling to Gαq are required for RhoA activation and subsequent stress fibre formation, indicating a convergence of the two pathways at the level of RhoA. AT1R, type I angiotensin II receptor; GPCR, G-protein-coupled receptor; MAPK, mitogen-activated protein kinase; PAR, protease-activated receptor.