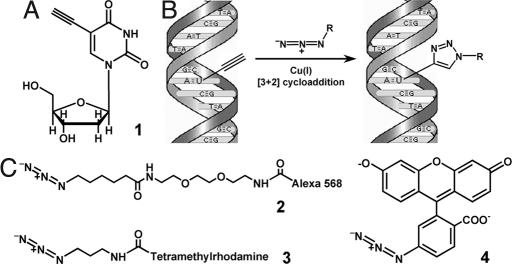
Fig. 1.
Use of 5-ethynyl-2′-deoxyuridine (EdU) to label DNA in cells. (A) Structure of 5-ethynyl-2′-deoxyuridine, a thymidine analogue that carries a terminal alkyne group instead of a methyl in the 5 position of the pyrimidine ring. (B) Schematic of the click reaction for detecting EdU incorporated into cellular DNA. The terminal alkyne group, exposed in the major groove of the DNA helix readily reacts with an organic azide (R can be any fluorophore, hapten, electron-dense particle, quantum dot, etc.) in the presence of catalytic amounts of Cu(I). (C) Structures of the fluorescent azides used in the present study. Unlike azide 2, azide 3 is cell-permeable, allowing cells to be stained while alive, without fixation and/or permeabilization. Fluorescein azide 4 can be easily prepared in large amounts by using inexpensive starting materials. See text and Materials and Methods for details.