Abstract
Seabirds are sensitive indicators of changes in marine ecosystems and might integrate and/or amplify the effects of climate forcing on lower levels in food chains. Current knowledge on the impact of climate changes on penguins is primarily based on Antarctic birds identified by using flipper bands. Although flipper bands have helped to answer many questions about penguin biology, they were shown in some penguin species to have a detrimental effect. Here, we present for a Subantarctic species, king penguin (Aptenodytes patagonicus), reliable results on the effect of climate on survival and breeding based on unbanded birds but instead marked by subcutaneous electronic tags. We show that warm events negatively affect both breeding success and adult survival of this seabird. However, the observed effect is complex because it affects penguins at several spatio/temporal levels. Breeding reveals an immediate response to forcing during warm phases of El Niño Southern Oscillation affecting food availability close to the colony. Conversely, adult survival decreases with a remote sea-surface temperature forcing (i.e., a 2-year lag warming taking place at the northern boundary of pack ice, their winter foraging place). We suggest that this time lag may be explained by the delay between the recruitment and abundance of their prey, adjusted to the particular 1-year breeding cycle of the king penguin. The derived population dynamic model suggests a 9% decline in adult survival for a 0.26°C warming. Our findings suggest that king penguin populations are at heavy extinction risk under the current global warming predictions.
Keywords: climate changes, seabirds, time lag, unbanded penguins
Predicting the impact of future climate changes on populations and biodiversity is a central issue in the context of global climate warming. It is, thus, crucial to understand how and to what extent organisms are able to cope with climatic variations, especially in polar environments where the effect of climate change is the strongest (1). Still, little is known about the effects of large-scale environmental perturbation on the productivity in the Circumpolar Austral Ocean (2). Most studies are using single weather variables such as sea surface temperature (SST) although the use of “weather packages” (3)[e.g., Southern Oscillation index (SOI)] should be called for (4). For instance, SST is commonly used as a proxy for food abundance and explanatory variable in studies of seabird population dynamic (5–7). Indeed, changes in SST in the Southern Ocean affect both marine productivity (8) and location of oceanographic structures used as feeding grounds (9, 10) by upper trophic-level predators, such as seabirds.
Here, we analyzed the effect of climate on subpolar seabird population by using a unique database on unbanded king penguins (Aptenodytes patagonicus) breeding at the Possession Island in the Crozet Archipelago marked by subcutaneous tags. A major interest of studying king penguins is that their breeding cycle extends over 12 months (11), which means that the birds are still breeding when marine resources are at their minimum and geographically more unpredictable. It is during such critical periods that even small environmental changes might have a serious impact on individual fitness components and ultimately at the population level. King penguins are moreover foraging both locally and remotely (12, 13), in opposition to other Antarctic seabirds (5, 6, 14), which gives a better coverage of the Southern Ocean and a shining example on how top predator populations hinging on this large ocean might be affected by global changes. In addition, the absence of flipper band in our study removes the now well documented cumulative detrimental effect on both Antarctic and Subantarctic penguins' breeding success (15) and survival (16). The environmental conditions affecting the penguin in our study are representative of those affecting the whole population, because ≈2/3 of the world's king penguin population breed on Crozet Archipelago (17). Hence, our long-term monitoring therefore provides a health state of this species.
To understand how stochasticity of the environment affects life-history strategies in populations, a simultaneous exploration of both patterns of variance in adult survival and breeding performances is necessary. According to life-history theory (18), adult survival is the parameter that has the highest elasticity in long-lived species. It is therefore expected to be the life-history trait in which an effect of climatic variability should be least apparent (19) in contrast to breeding parameters. In the context of life-history trade-offs, assuming that long-lived seabirds are unwilling to jeopardize their survival, we predicted that warm events might negatively affect the demographic parameters of upper-level predators, through modifications of food-web processes.
Results
Our time series corresponds to 9 years of data, which enabled us to obtain 6 years of annual breeding success and 8 years of adult survival (see Materials and Methods). Our analyses integrate different explanatory variables [see supporting information (SI) Table 3]. As a large-scale climatic variation index, we used SOI. As a local climate index, we used area-averaged SST and chlorophyll-a concentration [Chla] calculated in the area centered on Crozet Archipelago (43–47°S, 46–56°E), which corresponds to the most productive area of the region. In addition, mean SSTs were calculated every two degrees of latitude in the far-southern sector (48–60°S, 46–56°E), encompassing the southernmost foraging range in the vicinity of the winter pack ice (12). Most appropriate models, as found through Akaike's information criterion (AIC)-based model selection (Tables 1 and 2 and SI Table 4), show a negative effect of warming on both breeding success and survival.
Table 1.
Results of fitting linear models with binomial distribution to the variation of breeding success of king penguins from Crozet Islands
| Birds status | Explanatory variables |
ΔAIC* | Fixed effect,†P value | Random effect, P value | ANOVA, P value | |
|---|---|---|---|---|---|---|
| X1 | X2 | |||||
| Early breeders | SOIt | Val. [Chla]Cro t | 0 | <0.0001 | 0.002 | <0.0001 |
| SOIt | Val. [Chla]Cro t−1 | 8.43 | <0.0001 | 0.005 | 0.030 | |
| SOIt | Date [Chla]Cro t | 19.30 | <0.0001 | 0.006 | <0.0001 | |
| SOIt | Lat.SSTiso4°C t | 22.77 | <0.0001 | 0.001 | 0.001 | |
| SOIt | Date SSTCro t−2 | 26.50 | <0.0001 | 0.009 | 0.005 | |
| SOIt | Lat.SSTiso2°C t | 28.57 | <0.0001 | 0.001 | 0.016 | |
| SOIt | − | 32.38 | <0.0001 | 0.001 | ||
| Late breeders | SOIt | Val. SSTCro t−1 | 0 | 0.002 | 0.794 | 0.051 |
| SOIt | Lat.SSTiso2°C t | 0.55 | 0.001 | 0.618 | 0.071 | |
| SOIt | Date SSTCro t−2 | 0.78 | 0.001 | 0.717 | 0.081 | |
| SOIt | Lat.SSTiso4°C t | 0.96 | 0.003 | 0.675 | 0.091 | |
| SOIt | Date [Chla]Cro t−2 | 1.11 | 0.001 | 0.546 | 0.100 | |
| SOIt | − | 1.82 | 0.003 | 0.468 | ||
The explanatory variables are factors that might affect the breeding success. Best models are indicated in bold. SOI, annual value of the Southern Oscillation Index; Val. [Chla]Cro, highest value of chlorophyll; Date [Chla]Cro, date of Val. [Chla]Cro in the sector around Crozet (43°-47°S, 46°-56°E); Val. SSTCro, highest value of sea surface temperature; Date SSTCro date of Val. SSTCro for the Crozet sector; Lat.SSTiso4°C, location of the isotherm 4°C; Lat.SSTiso2°C location of the isotherm 2°C (corresponding to the Polar Front location; ref. 12).
*ΔAIC, difference in value between AIC of the most parsimonious model and the model in question. The year during which each variable was measured is indicated in subscript.
†Fixed effect, the effect of the explanatory variables. Random effect, the individual effect. The P values were obtained by using a parametric bootstrap of 1,000 replicates. ANOVAs compare the model with one explanatory variable (SOIt) to models with two variables (SOIt + X2).
Table 2.
Environmental effects on annual adult survival of king penguins from Crozet Islands
| Type/sector | Covariate | DEV | ΔAICi | NP | ER | R2 |
|---|---|---|---|---|---|---|
| Time-dependent survival | 1,422.84 | 9.43 | 8 | 0.0089 | 1.0000 | |
| South Crozet | SSTt−2 annual/56°S | 1,425.40 | 0.00 | 2 | 1.0000 | 0.9165 |
| South Crozet | SSTt−2 annual/54°S | 1,425.98 | 0.58 | 2 | 0.7501 | 0.8978 |
| South Crozet | SSTt−2 annual/58°S | 1,426.65 | 1.25 | 2 | 0.5354 | 0.8759 |
| South Crozet | SSTt−2 annual/52°S | 1,429.00 | 3.59 | 2 | 0.1659 | 0.7996 |
| South Crozet | SSTt−2 winter/48°S | 1,429.33 | 3.93 | 2 | 0.1404 | 0.7888 |
| South Crozet | SSTt−2 winter/52°S | 1,430.39 | 4.98 | 2 | 0.0828 | 0.7544 |
| South Crozet | SSTt−2 annual/60°S | 1,430.50 | 5.09 | 2 | 0.0784 | 0.7509 |
| Crozet | Mini SSTCro t−1 + spring/summer SSTCro t | 1,430.27 | 6.86 | 3 | 0.0323 | 0.7584 |
| Crozet | Mini SSTCro t−1 | 1,432.52 | 7.12 | 2 | 0.0285 | 0.6850 |
| Crozet | Mini SSTCro t−1 + SSTCro t at Chla bloom | 1,430.54 | 7.13 | 3 | 0.0283 | 0.7495 |
| South Crozet | SSTt−2 winter/54°S | 1,433.10 | 7.69 | 2 | 0.0213 | 0.6662 |
| South Crozet | SSTt−2 annual/50°S | 1,434.71 | 9.30 | 2 | 0.0095 | 0.6138 |
| Constant survival | 1,453.58 | 26.17 | 1 | 0.0000 | 0.0000 |
The best climatic models are shown. The model with the highest value of evidence ratio indicates the best model indicated in bold. DEV, deviance; NP, number of estimated parameters; ER, evidence ratio, i.e., the ratio of the AIC weights (wi) of the current model and the best model, equivalent to model likelihood given the set of models (the AIC weight of the best model was w0 = 0.231, any other wi can be found by multiplication of w0 with the model's evidence ratio); R2, variance explained; ΔAICi, Difference in value between AIC of the most parsimonious model and the model in question. SST (annual or winter) stands for mean values calculated at different latitudes. Mini SSTCro stands for the lowest value of SST in the sector around Crozet (43–47°S, 46–56°E). SSTCro at Chla bloom corresponds to the mean SST between October and December, and spring/summer SSTCro corresponds to the mean SST between September and April. SOI stands for the annual value of the Southern Oscillation Index. The year each variable was measured is indicated in subscript.
Breeding Analyses.
Independently of the timing of breeding, the best models (lowest AIC) predict that the breeding success of king penguin was negatively affected by low values of the annual mean SOI [or a warm phase of El Niño Southern Oscillation (ENSO)] in year t (Table 1). A model with both covariates SOI and [Chla] was preferred for birds breeding early in the season (ΔAIC = 32.38, Table 1). The chance of success in year t of early breeders was therefore positively related to the maximum [Chla] in the Crozet Archipelago sector in year t, whereas that of late breeders tended to be negatively affected by high SST (the previous year) in the same area and by the Polar Front position in year t. Nevertheless, despite the fact that models with the additive effect of SST had the lowest AIC, they did not provide more information than the retained model with the single SOI effect (ΔAIC <2, Table 1).
Survival Analyses.
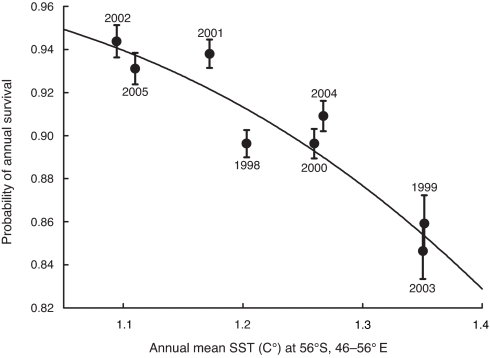
The overall goodness-of-fit test showed that the general Cormack–Jolly–Seber (CJS) model [pt φt] satisfactorily fitted our data (χ22 = 0.19, P = 0.91) and was used as a starting point for model selection. We detected no sex influence on the survival probability (SI Table 4). Survival was particularly low in 1999 and 2003 (0.8535, 95% CI = 0.8259–0.8774 and 0.8540, 95% CI = 0.8266–0.8777, respectively). Among the covariates examined (see SI Table 3), annual mean SST 2 years earlier at the latitude 56°S (SSTt− 2 annual/56°S) accounted for most yearly variations in survival (91.65%; Table 2). The constrained models integrating the annual SST at 54°S and 58°S, still with a time-lag of 2 years, were also supported by the data (ΔAICi <2; Table 2). Fewer king penguins survived when SSTs were higher two years earlier in the northern part of the marginal ice zone. The functional dependence between SSTt − 2 and the adult survival probability from the best model is shown in Fig. 1.
Fig. 1.
Effect of sea-surface temperature on the survival of adult king penguins. The probability of annual survival of adult king penguins from Crozet Islands plotted against the sea-surface temperature 2 years earlier at the latitude 56°S (i.e., MIZ). Estimates are from the time-dependent model [pi φt]. Bars indicate ± standard error. Plotted curve corresponds to the estimates obtained with the best model [pi φSSTt − 2/56°S].
Discussion
Climate fluctuations have important ecological consequences and influence the population dynamics of long-lived organisms (4). Environmental variables are certainly acting mainly indirectly on reproductive performance and survival of upper-level predators, such as penguins, and might affect the foraging efficiency of individuals through their effect on lower levels of the food web (e.g., refs. 20 and 21). Primary productivity is related to the local environmental conditions (8), such as temperature, wind, rain, snow, and ocean currents, as well as interactions among these. The relationship we found between the breeding success and the SOI (Table 1) might be explained by the fact that a combination of weather features (3) encompassed in a global indice such as SOI might be integrated at the higher trophic level (5). No time lag was found between annual breeding success and environmental parameters (Table 1), certainly because chick food depends mainly on the availability of resources produced close to the colony during the year. During the summer, king penguins are feeding principally on mesopelagic myctophids (22) abundant in the Antarctic Polar Frontal Zone (23) [(PFZ) i.e., 49–53° S]. In contrast, the majority of the winter diet is based on demersal onychoteuthid squid and neritic myctophid fish of the shelf waters (24). Myctophids and onychoteuthids feed on the meso- and macroplankton (25) whose fecundity and growth depend on phytoplankton production (26) and, hence, are sensitive to SST variation. Thus, the low breeding success observed in some years (Table 1) might result from the depletion of [Chla] (i.e., from a low phytoplankton production). The growth rate of zooplankton and larvae fishes is also known to be linked to a restricted thermal window, with an adverse effect of abnormally high SST (26). During the summer, an increase of the foraging trip duration of the breeder to reach the PFZ linked to warm events (negative SOI values) might (i) jeopardize the survival of newly hatched chicks and (ii) directly affect the parent's capacity to recover proper fuel reserves after the incubation fast, and it may accordingly choose to abandon reproduction in favor of its own survival (27). During the winter, king penguin's prey fish are found at a much greater depth in the PFZ than in summer (25) and are consequently unavailable for the birds which must therefore find an alternative resource. Accordingly, to feed their chick during winter, breeding penguins forage predominantly on small- and medium-sized juvenile squids spawned the previous spring (24) in the outer shelf, upper slope, and oceanic areas in the close vicinity of Crozet Islands (22). Low [Chla] around Crozet Archipelago, concomitant with negative SOI, might impact the fecundity and the growth of the squids, hence reducing the winter chick provisioning. The cumulative effect of a more distant PFZ location from the colony during the summer may lead to high reproductive failures in that specific year.
We show that the survival of king penguins is reduced when SST was high in the marginal ice zone (MIZ) 2 years earlier (Fig. 1 and Table 2). This reduction can be linked to prey populations at the northern boundary of pack ice. A poor sea ice cover the winter after a year with high SST might negatively affect in cascade each level of the food web (9, 28). Indeed, during the winter, breeding birds leave the PFZ foraging area to spend a substantial proportion of their time at sea below the northern sea ice limit (13) (56–57° S, i.e., MIZ). King penguin diet in this area is still unknown, but a study suggests that they compensate for the limited access to myctophids in winter by shifting their diet to Euphausia superba Krill species (13), on which they might rely to replenish their body fuels in distant areas (22) such as the MIZ. Consequently, 1 year after a warm event, king penguins might increase their energy expenditure for foraging (12) because of the poor prey abundance (krill or myctophid species) in the MIZ at the detriment of their survival the following year. This second-year lag may actually be explained by the delayed effect of costs occurring during the reproductive season (29) adjusted to the particular 1-year breeding cycle of the king penguin. Contrary to life-history theory expectation for a long-lived bird, breeding king penguins therefore seem to shunt efforts toward their offspring rather than for their own survival (see ref. 30).
Altogether, our data analysis, which avoids the bias of flipper banding, demonstrates that high SSTs have a detrimental effect on breeding and/or survival of king penguins. Moreover, we also show that the effect of warming on these two fundamental demographic traits is related to two different oceanographic processes linked to two foraging areas. Breeding success is related, without time lag, to the proximate environmental conditions in the north of the PFZ (at the Polar Front during the summer and over the Crozet shelf during the winter), whereas adult survival is linked, with a 2-year lag, to the winter SST in the remote MIZ. Importantly, and according to life-history theory in long-lived species (18), king penguin populations would not be sustained with a 9% drop in their adult survival such as that we show for an increase of only 0.26°C in SST (Fig. 1). Age might also be an important determinant of individual fitness and might have a potential impact on population growth rates (31, 32). Old birds (33) and first-time breeders (Constraint hypothesis; refs. 34 and 35) might be more sensitive to climatic variation than prime-age adults, as is known, e.g., for large ungulates (see ref. 36). Thus, at more long-term, our cohorts of 10-month-old chicks micro-tagged each year from 1998 will allow us to look at this age-related pattern of survival by analyzing these three groups separately. However, in line with the last Intergovernmental Panel on Climate Change (IPCC)-2007 estimation of a linear increasing trend of 0.74°C of global surface temperature during the last century and a further warming of ≈0.2°C per decade for the next two decades (37), the warming of the Southern Ocean certainly represents a major threat for penguins. Some other seabird communities in Antarctica might be affected by such a cascade of effects of Southern Ocean warming.
Materials and Methods
Species and Demographic Survey.
The specificity of the king penguin is that birds do not succeed in carrying out all breeding activities (courtship, egg laying, incubation, and chick fledging) within the summer period. Moreover, birds are not synchronized in their egg-laying date, and the laying period extends over 4 months, i.e., from November to the beginning of March. Two peaks of laying are observed: a first peak of early breeders which corresponds to those birds that did not breed or have failed in their previous breeding season, and a second peak of late breeders corresponding to those birds that have succeeded in fledging a chick during the previous breeding season (11). Such a unique breeding cycle appears to result from time-restricted access to resources, which fluctuate seasonally in abundance (22). Then, the favorable summer season is too short for penguins to complete moulting and breeding and at the same time produce a chick with good prospects of survival (38).
Our study was conducted at the Possession Island (46°25′S, 51°45′E, “La Grande Manchotière” colony) on Crozet Archipelago. From the austral summer 1997/1998 to 1999/2000, 456 king penguins were fitted as breeding birds with electronic tags without any other external mark. Antennas are permanently buried on their usual pathways and connected to a computerized reading system (39). The sequence of signals from the antennas reveals if a bird is entering or leaving the breeding site. This identification system does not require the bird's recapture or visual observation by a human and allows continuous automatic data collection (39). Our analysis excluded animals that had not reached reproductive age. The breeding cycles and parameters of the birds were established by interpreting the movements of the birds between the breeding area and the sea (see ref. 11 for details). Individual laying date and breeding success were recorded each year. Sex specificity is a common source of variation in survival probability of some birds, and it was necessary to determine preliminarily whether we needed to incorporate this variable in our models. The sex of the birds was determined by analyzing the chronology of the sex-specific incubating shifts (27).
Oceanographic Context and Environmental Descriptors.
SI Table 3 summarizes the data used and gives the different periods and time lags considered for each environmental variable.
Monthly SOI, daily SST (°C), and weekly [Chla] (mg.m−3) were obtained from the Australian Bureau of Meteorology, the National Ocean and Atmospheric Administration, and from the sea-viewing wide-field sensor, respectively. First, we extracted the monthly SOI values from August 1991 to October 2005. SOI was used as a proxy of a remote forcing to the local SST. Negative SOI values indicate a warm phase of ENSO (40), which are generally associated in our study area with warm SST anomalies (41). Monthly averages of SST were calculated from July 1995 until January 2006 in an area centered on Crozet Archipelago, bounded by 43–47°S, 46–56°E. This subsector corresponds to the yearly most productive subsector in the Crozet region, shallow shelves and weak currents acting as a retention zone (42), SST in this area might therefore influence the primary productivity. Similar calculations were performed for [Chla] data in this same subsector from September 1997 (first data available in this region) to April 2006. [Chla] is at the basis of food-web setting-up and thus might impact apex predators. Minimum SST, maximum SST, maximum [Chla], and their associated time were determined. Lower SSTs in spring and summer (high photoperiod) in this area might favor phytoplankton bloom, yielding high [Chla]. Moreover, monthly SSTs were calculated from July 1995 until January 2006 at every two latitudes in the area south of Crozet bounded by 48–60°S, 46–56°E, encompassing the southernmost foraging range (12). SST south of Crozet can influence the position of fronts, such as PFZ, and, consequently, the location of the trophic chain. Satellite tracking indicated that king penguins from this colony forage at the PFZ during summer in a sector comprising 48–52°S. In contrast, they cross the northern limit of the light pack ice in winter to forage at farther southern latitudes of 56–62°S (12).
Breeding Analyses.
The breeding success data (range from 0 to 1, obtained from 1998/1999 to 2003/2004) for each individual were analyzed by using a maximum likelihood mixed regression approach (43). Models fitted were linear ones with binomial distribution (i.e., generalized linear models with binomial distribution, using lme4 package for R 2.5.1 statistical program, ref. 44). Fixed effects (environmental variables) and random effects (individual) significance were tested by computing the likelihood ratio statistics on parametric bootstrap generated data (1,000 times) (43). We used ANOVAs to test the significance of adding explanatory variables and an information theoretic approach (45) to choose which of the environmental variables predicted a better breeding success. The most appropriate model was selected by using the AIC.
Adult Survival Analyses.
Data were analyzed in a capture–mark–recapture framework. Using the M-SURGE computer program (46), we estimated survival probabilities of adults from November 1997 to April 2006 (k = 9 occasions) by using the CJS models, which provide unbiased estimators of survival probabilities (47). All adults detected in a given summer had been recorded the previous summer. We therefore considered that the probability of detecting a bird (breeding or nonbreeding) provided it remained alive was equal to 1 (see ref. 48). Consequently, the reliability of survival estimates should be high (47), and this automatic identification system complies with the assumptions of the known-fate model. Winter survival was defined as the probability of survival from summer t (November of year t to May of year t + 1) to summer t + 1 (November of year t + 1 to May of year t + 2). We used the goodness-of-fit tests computed by program U-CARE (49) to check that our most general model fitted the data. Then, we used AIC to select the best model in terms of both parsimony (fewest model parameters) and adequate description of the data. Models with the lowest values of AIC were retained as good candidate models (47). Because AIC values are interpretable only in terms of “relative value,” we relied on AIC weights (w) to select models when the difference between the AIC values of two models was lower than 2 (47). We then modeled the yearly variations in adult survival as a function of covariates that might be relevant to the ecology of king penguins (SI Table 3). To assess the effects of these environmental variables, the amount of variations accounted for covariates, i.e., the direct estimation and quantification of the relative effects of environmental variability on survival (50), was calculated by using an analysis of deviance (R2; ref. 45), which is defined as [DEV(constant model) − DEV(covariate model))/(DEV(constant model) − DEV(time-dependent model)]. This corresponds to the proportion of explained variance and is comparable to a squared correlation coefficient.
Supplementary Material
ACKNOWLEDGMENTS.
We thank I. Durant, F. Roquet, C. Lesage, J.-B. Charrassin, C. Salmon, and V. Viera for their help in analyzing data or their constructive comments on the manuscript; and M. Ballesteros, D. Beaune, C. Bricaud, N. Chatelain, G. Conan, S. Descamps, J. Dutel, C. Gilbert, S. Gravier, A. Hergott, G. Kuntz, N. Lambert, N. Lecomte, J. Legrand, S. Mangin, V. Mosch, S. Quéméneur, A. Simon, E. Taquet, and C. Villemin for their help on field work. This work was supported by the Institut Polaire Français–Paul-Emile Victor (IPEV), the Centre National de la Recherche Scientifique, Programme Zone Atelier de Recherches sur l'Environnement Antarctique et Subantarctique, and grants from the Institut de France and the Fondaton des Treilles (to C.L.B.) and from the Norwegian Research Council through the Marine Diversity Management (MaDiMa) project (to J.M.D.)
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0712031105/DC1.
References
- 1.Clarke A, Harris CM. Polar Marine Ecosystems: Major Threats and Future Change. Cambridge, UK: Cambridge Univ Press; 2003. [Google Scholar]
- 2.McMahon CR, Burton HR. Climate change and seal survival: Evidence for environmentally mediated changes in elephant seal, Mirounga leonina, pup survival. Proc R Soc London Ser B. 2005;272:923–928. doi: 10.1098/rspb.2004.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stenseth NC, et al. Studying climate effects on ecology through the use of climate indices, the North Atlantic Oscillation, El Niño Southern Oscillation and beyond. Proc R Soc London Ser B. 2003;270:2087–2096. doi: 10.1098/rspb.2003.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stenseth NC, et al. Ecological effects of climate fluctuations. Science. 2002;297:1292–1296. doi: 10.1126/science.1071281. [DOI] [PubMed] [Google Scholar]
- 5.Croxall JP, Trathan PN, Murphy EJ. Environmental change and Antarctic seabirds populations. Science. 2002;297:1510–1514. doi: 10.1126/science.1071987. [DOI] [PubMed] [Google Scholar]
- 6.Barbraud C, Weimerskirch H. Emperor penguins and climate change. Nature. 2001;411:183–186. doi: 10.1038/35075554. [DOI] [PubMed] [Google Scholar]
- 7.Veit RR, McGowan JA, Ainley DG, Wahls TR, Pyle P. Apex marine predator declines ninety percent in association with changing oceanic climate. Glob Change Biol. 1997;2:23–28. [Google Scholar]
- 8.Gregg WW, Conkright ME, Ginoux P, O'Reilly JE, Casey NW. Ocean primary production and climate: Global decadal changes. Geophys Res Lett. 2003;30:1809. [Google Scholar]
- 9.Nicol S, et al. Ocean circulation off east Antarctica affects ecosystem structure and sea ice extent. Nature. 2000;406:504–507. doi: 10.1038/35020053. [DOI] [PubMed] [Google Scholar]
- 10.Moore JK, Abbott MR, Richman J. Location and dynamics of the Antarctic Polar Front from satellite sea surface temperature data. Geophys Res Lett. 1999;104:3059–3073. [Google Scholar]
- 11.Descamps S, Gauthier-Clerc M, Gendner J-P, Le Maho Y. The annual breeding cycle of unbanded king penguins Aptenodytes patagonicus on Possession Island (Crozet). Avian Sci. 2002;2:87–98. [Google Scholar]
- 12.Charrassin J-B, Bost CA. Utilisation of the oceanic habitat by king penguins over the annual cycle. Mar Ecol Prog Ser. 2001;221:285–297. [Google Scholar]
- 13.Bost CA, Charrassin J-B, Clerquin Y, Ropert-Coudert Y, Le Maho Y. Exploitation of distant marginal ice zones by king penguins during winter. Mar Ecol Prog Ser. 2004;283:293–297. [Google Scholar]
- 14.Jenouvrier S, Barbraud C, Weimerskirch H. Sea ice affects the population dynamics of Adélie penguins in Terre Adélie. Polar Biol. 2006;29:413–423. [Google Scholar]
- 15.Gauthier-Clerc M, et al. Long-term effects of flipper bands on penguins. Proc R Soc London Ser B. 2004;271:S423–S426. doi: 10.1098/rsbl.2004.0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dugger KM, Ballard G, Ainley DG, Barton KJ. Effect of flipper-bands on the foraging behavior and survival of Adélie Penguins in the southern Ross Sea. Auk. 2006;123:858–869. [Google Scholar]
- 17.Guinet C, Jouventin P, Malacamp J. Satellite remote sensing in monitoring change of seabirds: Use of SPOT Image in King Penguin population increase at Ile aux Cochons, Crozet Archipelago. Polar Biol. 1995;15:511–515. [Google Scholar]
- 18.Stearns SC. The Evolution of Life Histories. Oxford: Oxford Univ Press; 1992. [Google Scholar]
- 19.Gaillard J-M, Yoccoz NG. Temporal variation in survival of mammals: A case of environmental canalization? Ecology. 2003;84:3294–3306. [Google Scholar]
- 20.Durant JM, et al. Marine birds and climate fluctuations in the North Atlantic. In: Stenseth NC, Ottersen G, Hurrell JW, Belgrano A, editors. Marine Ecosystems and Climate Variation: The North Atlantic. Oxford: Oxford Univ Press; 2004. pp. 95–105. [Google Scholar]
- 21.Durant JM, Anker-Nilssen T, Stenseth NC. Ocean climate prior to breeding affects the duration of the nestling period in the Atlantic puffin. Biol Lett. 2006;2:628–631. doi: 10.1098/rsbl.2006.0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cherel Y, Verdon C, Ridoux V. Seasonal importance of oceanic myctophids in king penguin diet at Crozet Island. Polar Biol. 1993;13:355–357. [Google Scholar]
- 23.Duhamel G, Koubbi P, Ravier C. Day and night mesopelagic fish assemblages off the Kerguelen Islands (Southern Ocean). Polar Biol. 2000;23:106–112. [Google Scholar]
- 24.Cherel Y, Weimerskirch H. Spawning cycle of onychoteuthid squids in the southern Indian Ocean: New information from seabird predators. Mar Ecol Prog Ser. 1999;188:93–104. [Google Scholar]
- 25.Koslov AN, Tarverdiyevma I. Feeding of different species of Myctophidae in different parts of the Southem Ocean. J Ichthyol. 1989;29:160–167. [Google Scholar]
- 26.Richardson AJ, Verheye HM. The relative importance of food and temperature to copepod egg production and somatic growth in the southern Benguela upwelling system. J Mar Sci. 1998;55:803–807. [Google Scholar]
- 27.Gauthier-Clerc M, Le Maho Y, Clerquin Y, Bost C-A, Handrich Y. Seabird reproduction in an unpredictable environment: how king penguins provide their young chicks with food. Mar Ecol Prog Ser. 2002;237:291–300. [Google Scholar]
- 28.Fraser WR, Hofmann EE. A predator's perspective on causal links between climate change, physical forcing and ecosystem response. Mar Ecol Prog Ser. 2003;265:1–15. [Google Scholar]
- 29.Williams TD. The Penguins. Oxford: Oxford Univ Press; 1995. [Google Scholar]
- 30.Olsson O, van der Jeugd HP. Survival in king penguins Aptenodytes patagonicus: temporal and sex-specific effects of environmental variability. Oecologia. 2002;132:509–516. doi: 10.1007/s00442-002-0985-6. [DOI] [PubMed] [Google Scholar]
- 31.Charlesworth B. Evolution in age-structured populations. 2nd Ed. Cambridge, UK: Cambridge Univ Press; 1994. [Google Scholar]
- 32.Caswell H. Matrix Population Models. Sunderland, MA: Sinauer; 2001. [Google Scholar]
- 33.Williams GT. Natural selection, the costs of reproduction, and a refinement of Lack's principle. Am Nat. 1966;100:687–690. [Google Scholar]
- 34.Forslund P, Pärt T. Age and reproduction in birds-hypotheses and tests. Trends Ecol Evol. 1995;10:374–378. doi: 10.1016/s0169-5347(00)89141-7. [DOI] [PubMed] [Google Scholar]
- 35.Martin K. Patterns and mechanisms for age-dependent reproduction and survival in birds. Am Zool. 1995;35:340–348. [Google Scholar]
- 36.Gaillard J-M, Festa-Bianchet M, Yoccoz NG, Loison A, Toïgo C. Temporal variation in fitness components and population dynamics of large herbivores. Annu Rev Ecol Syst. 2000;31:367–393. [Google Scholar]
- 37.IPCC. Climate Change 2007. The Physical Science Basis. Summary for Policymakers of the Intergovernmental Panel on Climate Change. (WG I, 2007, available from www.ipcc.ch)
- 38.Weimerskirch H, Stahl J-C, Jouventin P. The breeding biology and population dynamics of King Penguin Aptenodytes patagonicus on the Crozet Islands. Ibis. 1992;134:107–117. [Google Scholar]
- 39.Gendner J-P, Gauthier-Clerc M, Le Bohec C, Descamps S, Le Maho Y. A new application for transponders in studying of penguins. J Field Ornitho. 2005;76:138–142. [Google Scholar]
- 40.Deser C, Wallace JM. El Niño events and their relation to the southern oscillation: 1925–1986. Geophys Res Lett. 1987;92:14189–14196. [Google Scholar]
- 41.Park Y-H, Roquet F, Vivier F. Quasi-stationary ENSO wave signals versus the Antarctic Circumpolar Wave scenario. Geophys Res Lett. 2004;31:L09315. [Google Scholar]
- 42.Perissinotto R, Duncombe Rae CM. Occurrence of anti-cyclonic eddies on the Prince Edward Plateau (Southern Ocean): Effects on phytoplankton biomass and production. Deep Sea Res. 1990;37:777–793. [Google Scholar]
- 43.Pinheiro JC, Bates DM. Mixed-Effects Models in S, S-PLUS. New York: Springer; 2000. [Google Scholar]
- 44.Bates D, Sarkar D. lme4: Linear Mixed-Effects Models Using S4 Classes. [Accessed January 25, 2007];2006 (R package version 0.9975-10). Available at: http://cran.r-project.org/src/contrib/Descriptions/lme4.html.
- 45.Burnham KP, Anderson DR. Model Selection and Multimodel Inference. New York: Springer; 2002. [Google Scholar]
- 46.Choquet R, Reboulet A-M, Pradel R, Gimenez O, Lebreton J-D. M-SURGE: New software specifically designed for multistate capture-recapture models. Anim Biodiv Cons. 2004;27:207–215. [Google Scholar]
- 47.Lebreton J-D, Burnham KP, Clobert J, Anderson DR. Modeling survival and testing biological hypotheses using marked animals: A unified approach with case studies. Ecol Monogr. 1992;62:67–118. [Google Scholar]
- 48.Le Bohec C, et al. Population dynamics in a long-lived seabird I: Impact of breeding activity on survival and breeding probability in unbanded king penguins. J Anim Ecol. 2007;76:1149–1160. doi: 10.1111/j.1365-2656.2007.01268.x. [DOI] [PubMed] [Google Scholar]
- 49.Choquet R, Reboulet A-M, Lebreton J-D, Gimenez O, Pradel R. [Accessed January 25, 2007];U-Care 2.2 (Utilities-Capture-Recapture) User's Manual. 2005 Available at: www.cefe.cnrs.fr/BIOM/PDF/Choquet-USER%20MANUAL%20U-CARE%202.2.pdf/
- 50.Gaillard JM, et al. Early survival in roe deer: Causes and consequences of cohort variation in two contrasted populations. Oecologia. 1997;112:502–513. doi: 10.1007/s004420050338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.