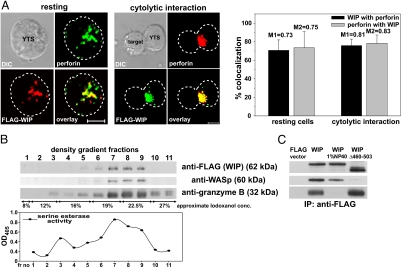
Fig. 1.
WIP is associated with lytic granules in NK cells. (A) Colocalization of FLAG-WIP and perforin in resting (Left) and activated (Center) YTS cells. YTS cells expressing FLAG-WIP, unconjugated or conjugated by mixing for 10 min at 37°C with 721.221 target cells, were stained with Cy3-conjugated anti-FLAG mAb (red) and AlexaFluor 647-conjugated anti-perforin (green) antibodies. (Scale bar: 5 μm.) The graph (Right) displays the percentage of FLAG-WIP colocalizing with perforin (black bars) and perforin that colocalized with FLAG-WIP (gray bars) in either resting or activated NK cells (n ≥ 10). Values above each bar represent Manders' colocalization coefficients. Error bars represent SD. (B) WIP and WASp are found in the lysosomal fraction of NK cells. YTS cells expressing FLAG-WIP were homogenized and fractionated by centrifugation, and crude lysosomal fraction was resolved on an 8–27% Iodoxanol density gradient. Proteins from gradient fractions were resolved on a NuPage gel, transferred to a PVDF membrane, and subsequently immunobloted with anti-FLAG, anti-WASp, and anti-granzyme B antibodies. The serine esterase activity (a marker for granzyme activity) was determined in each density gradient fraction and displayed graphically below. (C) WIP association with lytic granules is independent of WASp and requires intact granules. YTS cells, transfected with either FLAG-WIP or FLAG-WIPΔ460–503 or FLAG expression vector alone, were lysed by homogenization. Crude lysosomal fractions either nontreated or treated with 1% Nonidet P-40 (to solubilize cell membranes) were immunoprecipitated with anti-FLAG mAb. Immunoprecipitated proteins were resolved on a NuPage gel, transferred to a PVDF membrane, and immunobloted with anti-FLAG, anti-WASp, and anti-granzyme B antibodies. The molecular masses of the proteins, relative to masses of molecular markers, are shown in parentheses.