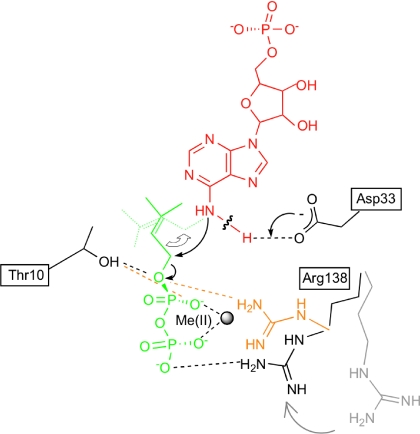
Fig. 4.
Proposed chemical reaction model of prenyl transfer from DMAPP to AMP in Tzs. Prenyl transfer proceeds by the SN2-nucleophilic displacement reaction. AMP is shown in red, DMAPP in green, and the amino acid residues of Tzs in black. Arg-138 in the absence of divalent metal ion [Me(II)] is shown in orange, and in the absence of prenyl-donor substrate in gray. The hydrogen-bonding network among the substrates and Thr-10, Asp-33, Arg-138, and Me(II) found in the Tzs-AMP/DMASPP/Zn and Tzs-AMP/DMASPP is indicated as a dashed line. In this model, the carboxylate group of Asp-33 serves as a general base to deprotonate the N6-amino group of AMP. The resulting nucleophile attacks the carbon (C1) of DMAPP. Collapse of the penta-covalent transition state yields the products N6-(Δ2-isopentenyl)adenine riboside 5′-monophosphate and diphosphate. Thr-10 and Arg-138 stabilize the penta-covalent transition state.