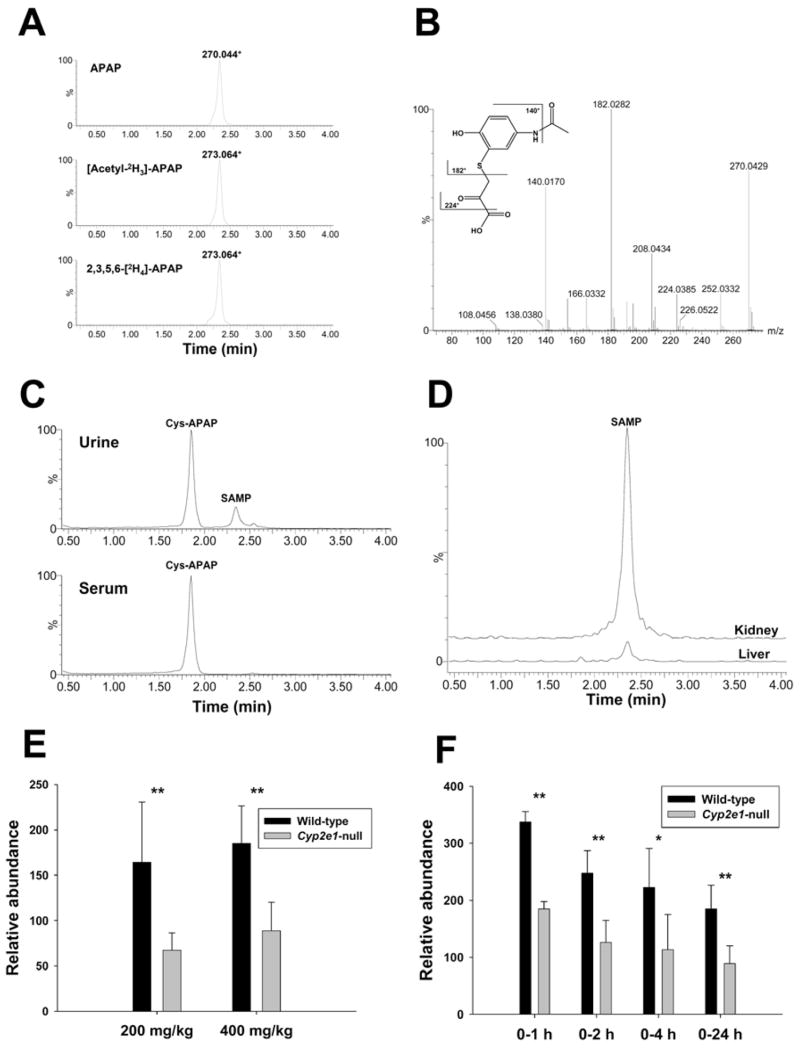
Figure 5.
Identification and characterization of metabolite VI as S-(5-acetylamino-2-hydroxyphenyl)mercaptopyruvic acid (SAMP). A. Presence of metabolite XI in the urine of wild-type mice treated with 400 mg/kg APAP, [acetyl-2H3]-APAP and 2,3,5,6-[2H4]-APAP. Ion chromatograms were prepared by extracting ions in the 20 ppm mass range of 270.044+ (APAP), 273.064+ ([acetyl-2H3]-APAP) and 273.064+ (2,3,5,6-[2H4]-APAP), respectively. B. MS2 spectrum of metabolite VI. Major fragment ions were interpreted in the inlaid structural diagrams. C. Comparison of the presence of Cys-APAP and SAMP in the urine and serum from APAP treatment. Ion chromatograms were prepared by extracting ions in the 20 ppm mass range of Cys-APAP (271.075+) and SAMP (270.044+). D. In vitro transamination reaction converting Cys-APAP to SAMP. Overlayed ion chromatograms from the reaction between liver/kidney homogenates and Cys-APAP were prepared by extracting ions in the 20 ppm mass range of SAMP (270.044+) and scaled by the counts of SAMP ion (the counts from kidney-mediated reaction was used as 100%). E. Relative abundances of metabolite VI in the 24-h urine of wild-type and Cyp2e1-null mice treated with 200 and 400 mg/kg APAP (n=8). F. Relative abundances of metabolite VI in the 1-h, 2-h, 4-h and 24-h urine of wild-type and Cyp2e1-null mice treated with 400 mg/kg APAP. Relative abundances of metabolite VI (mean ± SD in parts per ten thousand) were determined by normalizing the single ion counts of metabolite VI versus the total ion counts of each urine sample (n=4 for 1-h, 2-h, 4-h urine, * for P < 0.05 and ** for P < 0.01).