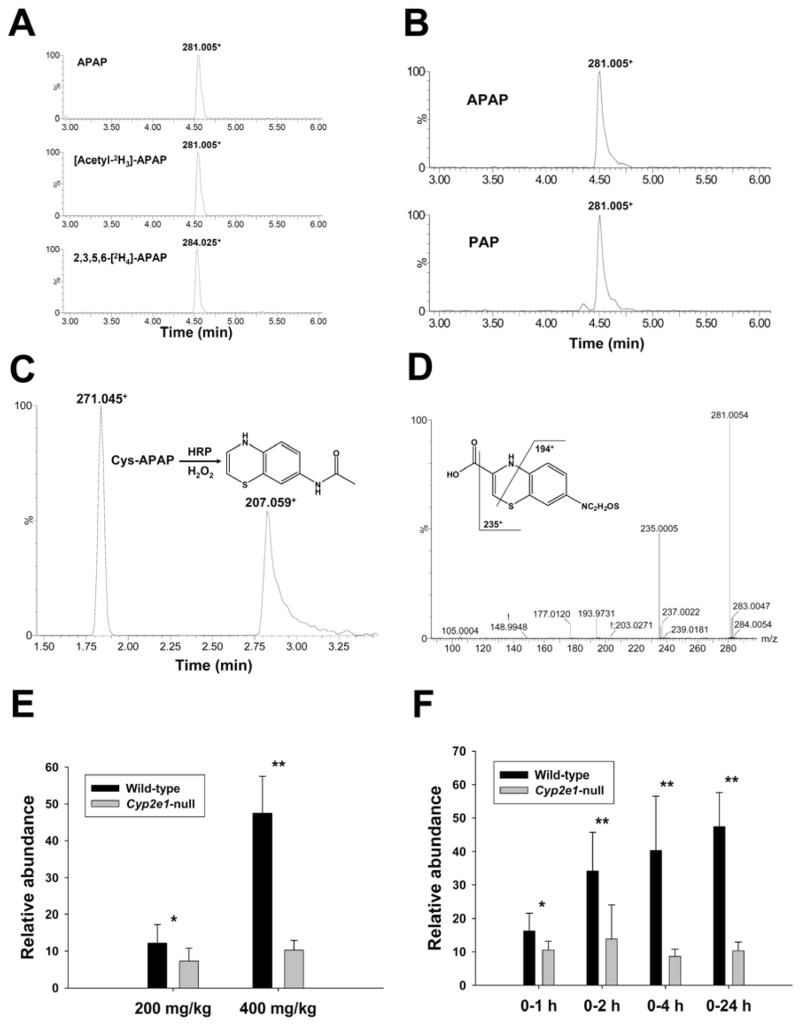
Figure 8.
Identification and characterization of metabolite IX as a benzothiazine compound. A. Presence of metabolite IX in the urine of wild-type mice treated with 400 mg/kg APAP, [acetyl-2H3]-APAP and 2,3,5,6-[2H4]-APAP. Ion chromatograms were prepared by extracting ions in the 20 ppm mass range of 281.005+ (APAP), 281.005+ ([acetyl-2H3]-APAP) and 284.025+ (2,3,5,6-[2H4]-APAP), respectively. B. Presence of metabolite IX in the urine of wild-type mice treated with 400 mg/kg p-aminophenol (PAP). Peaks corresponding to metabolite IX were labeled in the extracted ion chromatograms of PAP (lower panel) and APAP (upper panel) treatments. C. MS2 spectrum of metabolite IX. Major fragment ions were interpreted in the inlaid structural diagrams. D. LC-MS analysis of the products from the HRP-mediated reaction between Cys-APAP and H2O2. Peaks corresponding to Cys-APAP (271.045+) and N-acetylamino-1,4-benzothiazine (207.059+) were labeled in the ion chromatogram of the reaction. E. Relative abundances of metabolite IX in the 24-h urine of wild-type and Cyp2e1-null mice treated with 200 and 400 mg/kg APAP (n=8). F. Relative abundances of metabolite IX in the 1-h, 2-h, 4-h and 24-h urine of wild-type and Cyp2e1-null mice treated with 400 mg/kg APAP. Relative abundances of metabolite IX (mean ± SD in parts per ten thousand) were determined by normalizing the single ion counts of metabolite IX versus the total ion counts of each urine sample (n=4 for 1-h, 2-h, 4-h urine, * for P < 0.05 and ** for P < 0.01).