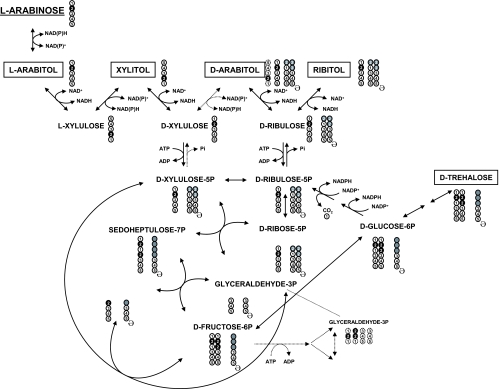
FIG. 5.
Schematic representation of l-arabinose metabolism and pattern of carbon distribution as determined by 13C NMR, HPLC, and chiral GC-MS. l-[2-13C]arabinose is metabolized through the redox catabolic pathway to d-[2-13C]xylulose-5-P. d-Xylulose-5-P proceeds through the PPP or is further converted to d-ribulose, yielding d-arabitol and ribitol. The reactions through the PPP yield fructose-6-P labeled in C-2 and C-3. C-3 labeling is specifically obtained with the carbon transfer (two carbons) from d-xylulose-5-P to d-erythrose-4-P. Fructose-6-P, labeled in C-2 and C-3, either enters the glycolytic pathway or is isomerized to glucose-6-P, the precursor of trehalose. In addition, glucose-6-P follows the oxidative PPP with decarboxylation and conversion to d-ribulose-5-P. The cycle is closed with the decarboxylation, and new species labeled in C-1 are produced (arabitol and trehalose). Extra cycles will maintain the same pattern of carbon labeling. The circles represent individual carbon atoms, numbered according to their position in the original l-[2-13C]arabinose. For each metabolite, filled circles represent 13C labeling. Gray circles represent all carbon positions that can become 13C enriched after carbon recycling (ə;) when l-[2-13C]arabinose is metabolized. Dashed lines indicate putative pathways.