Abstract
The development of the nervous system requires the concerted actions of multiple transcription factors, yet the molecular events leading to their expression remain poorly understood. Barhl1, a mammalian homeodomain transcription factor of the BarH class, is expressed by developing inner ear hair cells, cerebellar granule cells, precerebellar neurons, and collicular neurons. Targeted gene inactivation has demonstrated a crucial role for Barhl1 in the survival and/or migration of these sensory cells and neurons. Here we report the regulatory sequences of Barhl1 necessary for directing its proper spatiotemporal expression pattern in the inner ear and central nervous system (CNS). Using a transgenic approach, we have found that high-level and cell-specific expression of Barhl1 within the inner ear and CNS depends on both its 5′ promoter and 3′ enhancer sequences. Further transcriptional, binding, and mutational analyses of the 5′ promoter have identified two homeoprotein binding motifs that can be occupied and activated by Barhl1. Moreover, proper Barhl1 expression in inner ear hair cells and cerebellar and precerebellar neurons requires the presence of Atoh1. Together, these data delineate useful Barhl1 regulatory sequences that direct strong and specific gene expression to inner ear hair cells and CNS sensory neurons, establish a role for autoregulation in the maintenance of Barhl1 expression, and identify Atoh1 as a key upstream regulator.
The mammalian Barhl1 and Barhl2 proteins belong to the BarH class of homeodomain transcription factors that are evolutionarily conserved in both invertebrate and vertebrate species ranging from Drosophila flies to humans. The factors of this class are usually expressed in the developing and adult nervous systems and are required for proper neural development and survival (4, 7, 12, 13, 15, 18-20, 24, 26-32, 34). In Drosophila, BarH1 and BarH2 are coexpressed in cells of the central nervous system (CNS) and the peripheral nervous system and are redundantly required for determining the subtypes of external sensory organs and photoreceptor and primary pigment cells during eye development (12, 13, 15). The vertebrate Barhl1 and Barhl2 genes similarly display overlapping yet distinct patterns of expression in the CNS and sensory organs, thereby also playing redundant as well as distinct roles during neurogenesis (4, 7, 18-20, 24, 26-29, 31, 32, 34). For instance, Xenopus Barhl2 is expressed in the developing neural plate and retina during Xenopus embryogenesis and plays a key role in patterning the neural plate and specifying retinal ganglion cells (27, 29).
The functions of mammalian Barhl genes during murine neurogenesis have been extensively investigated previously. In the developing mouse inner ear, Barhl1 but not Barhl2 is specifically expressed in all hair cells of the cochlear and vestibular systems (4, 18). The targeted disruption of Barhl1 leads to hearing loss as a result of the age-related progressive degeneration of inner and outer hair cells in the organ of Corti (18), demonstrating a critical requirement for Barhl1 in the long-term maintenance of these sensory cells. In the developing CNS, Barhl1 and Barhl2 are expressed in the superior and inferior colliculi of the midbrain, the cerebellar granule cells and precerebellar neurons of the hindbrain, and the dorsal interneurons of the spinal cord (4, 19, 20, 26, 31, 32). Analyses of Barhl1-deficient mice have revealed a crucial role for Barhl1 in the migration and survival of cerebellar and precerebellar neurons and the long-term maintenance of superior collicular neurons as well (19, 20). In the spinal cord, Barhl1 inactivation results in no appreciable change, yet gain-of-function studies have implied a role for Barhl2 in the differentiation of dorsal commissural sensory neurons (19, 31, 32), suggesting a likely functional redundancy between Barhl1 and Barhl2 during spinal neurogenesis. In the developing retina, contrary to the inner ear, Barhl2 instead of Barhl1 is uniquely expressed in several inner retinal cell types and plays a role in the specification of glycinergic amacrine cells (26).
It is now clear that the exact functions of Barhl1 and Barhl2 factors depend to a large extent on the timing and location of their expression and activities. Thus, due to their distinct expression profiles, Barhl1 has a key role in maintaining cochlear hair cells whereas Barhl2 plays a role in specifying a retinal cell subtype (18, 26). Without doubt, proper spatial and temporal regulation of Barhl1 and Barhl2 expression is crucial for their normal function. At present, however, little is known about the molecular basis leading to their appropriate expression patterns, despite their essential roles during sensorineural development. A recent transgenic analysis of Barhl2-flanking sequences identified a 3′ enhancer that can be activated by the basic helix-loop-helix factor Atoh1/Math1 and can drive spinal cord-specific gene expression (31). In this study, we aimed to define Barhl1 regulatory sequences specific to inner ear hair cells and CNS areas. We found by transgenic analysis that 4.2-kb 5′ and 3.4-kb 3′ flanking sequences together are sufficient for recapitulating the endogenous Barhl1 expression pattern. The 4.2-kb promoter sequence is capable of driving specific gene expression to inner ear hair cells, the spinal cord, and the hindbrain, but high-level and midbrain-specific expression depends on a distal 1.7-kb 3′ enhancer sequence. Furthermore, Barhl1 and Barhl2 are able to auto- and cross-activate the Barhl1 promoter by binding to two specific homeoprotein binding sites, and Atoh1 is directly and/or indirectly required for proper Barhl expression in the inner ear and CNS.
MATERIALS AND METHODS
Plasmid constructs.
The transgenes were constructed by the excision of the Barhl1 5′ arm-lacZ sequence from an earlier Barhl1 knockout intermediate construct, pSDKlacZpA-5′ arm (18), by using the NotI and XhoI restriction endonucleases. The released fragment was joined with a 3.4-kb 3′ Barhl1-flanking sequence to yield the transgene construct Tg1 or a 1.7-kb proximal 3′ flanking sequence to generate Tg2 or was not fused to any 3′ flanking sequence to yield Tg3. Barhl1 and Barhl2 expression plasmids were constructed by inserting the full-length Barhl1 and Barhl2 cDNAs into the pcDNA 3.1 expression vector (Invitrogen). To make the R1 luciferase reporter construct, the 4.2-kb 5′ promoter sequence (bp −4246 to −1 relative to the translation start site) was excised from the Tg1 transgene by using the KpnI and XhoI restriction enzymes and then ligated into the pGL3-Basic vector (Promega). The R5 luciferase reporter plasmid was constructed by the excision of the 4.2-kb promoter sequence from R1 by using the KpnI and XhoI restriction enzymes, followed by ligation into the pGL3-Promoter vector (Promega). All other luciferase reporter constructs were derived from R1 or R5 plasmids by restriction digestion or PCR. The mutant constructs R10 to R12 were derived from R1 using PCR-based site-directed mutagenesis.
Generation of transgenic mouse lines.
Transgenic mice were generated by standard procedures (14, 40). In brief, linear DNA fragments of transgenic constructs were purified by gel electrophoresis and microinjected into pronuclei of C57BL/6J × FVB/N zygotes. Embryos were transplanted into foster mothers, and founder animals were identified by PCR analysis. The founders were crossed with C57BL/6J mice to produce F1 progeny, which were subjected to histochemical and immunostaining analyses. The following PCR primers for lacZ were used for genotyping: 5′GGTTGTTACTCGCTCACATTTAATG3′ and 5′CCATGCAGAGGATGATGCTCGTGAC3′.
X-Gal staining, in situ hybridization, and immunostaining.
X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining of whole-mount embryos and brains as well as cryosections was carried out as described previously (18, 19). Following X-Gal staining, some whole-mount embryos were dehydrated in graded ethanol and cleared in 1:2 benzyl alcohol-benzyl benzoate and some sections were counterstained with Fast Red (Vector Laboratories, CA).
Barhl1 in situ hybridization and immunostaining were carried out as described previously (19). The preparation of inner ear sections and the labeling of these sections by a double-immunofluorescence method were also performed as described previously (26, 39). The following primary and secondary antibodies were used for signal visualization: anti-myosin VI (anti-Myo6 [goat polyclonal antibody; Santa Cruz Biotechnology]), anti-β-galactosidase (anti-β-Gal [rabbit polyclonal antibody; Cappel]), Alexa Fluor 594-conjugated donkey anti-rabbit immunoglobulin G (IgG; Molecular Probes), and Alexa Fluor 488-conjugated donkey anti-goat IgG (Molecular Probes). DAPI (4′,6-diamidino-2-phenylindole; Vector Labs) was used for nuclear counterstaining.
Cell cultures and luciferase assays.
Cell cultures and luciferase assays were carried out by following modified versions of previously described procedures (23). Briefly, 293T cells were cultured in Dulbecco's modified Eagle's medium containing 10% fetal calf serum at 37°C and 5% CO2. Transfections of 105 cells were carried out by lipofection in six-well plates using the Lipofectamine reagent according to the instructions of the manufacturer (Invitrogen). Cells were cotransfected with 100 ng each of reporter and expression constructs, along with 25 ng of the pRL-TK renilla luciferase reporter plasmid (Promega) for the internal control of cell transfection efficiency. Cell extracts were assayed for firefly and renilla luciferase activities 48 h after transfection by using a dual luciferase assay system (Promega). All values of firefly luciferase activities were normalized with those of the corresponding renilla luciferase activities derived from the control plasmid. Experiments were performed in triplicate and repeated three times.
EMSAs.
Electrophoretic mobility shift assays (EMSAs) were carried out using in vitro-translated Barh1 and Barhl2 proteins as previously described (23). The protein products were generated by a TNT T7 or Sp6 coupled reticulocyte lysate system (Promega) using Barhl1 and Barhl2 expression plasmids. Protein synthesis was confirmed on sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis gels. DNA oligonucleotides were annealed, end radiolabeled with [γ-32P]ATP, and then purified using Bio-Spin 6 columns (Bio-Rad) for probes. Binding reactions were carried out at room temperature for 15 min in 1× buffer (10 mM HEPES [pH 7.5], 50 mM KCl, 1 mM EDTA, 0.1% Triton X-100, 5% glycerol, 0.1 mM dithiothreitol, and 0.1 mM phenylmethylsulfonyl fluoride) with 1 μg of poly(dI-dC), labeled probes in amounts corresponding to 5 × 105 cpm, and 4 μl of the desired protein lysates. Competition was performed by adding excess amounts of cold oligonucleotides to the reaction mixtures. Free and bound probes were resolved on a 5% nondenaturing polyacrylamide gel.
ChIP assays.
Chromatin immunoprecipitation (ChIP) assays were performed using the ChIP assay kit essentially according to the protocol of the manufacturer (Upstate Biotechnologies). In brief, to prepare chromatin DNA from cultured 293T cells, 1.5 × 107 cells were used 48 h after transfection with appropriate reporter and expression constructs. A cross-linking reaction was carried out with 1% formaldehyde for 10 min at room temperature and stopped by adding glycine to a final concentration of 0.125 M. Cells were then washed with phosphate-buffered saline (PBS) buffer, pelleted in a conical tube for 4 min at 2,000 rpm (IEC centra CL2), and resuspended in 1 ml of SDS lysis buffer. After the division of the lysate into 200-μl aliquots, the chromatin was sheared by sonication to an average size of between 200 and 1,000 bp. The debris was removed by centrifuging at 13,000 rpm (Eppendorf 5415C) for 10 min, and then the supernatant was diluted by the addition of 700 μl of ChIP dilution buffer. To reduce the nonspecific background, the diluted supernatant was precleaned with 75 μl of protein A-agarose beads for 30 min at 4°C. Following a brief centrifugation step, the supernatants were rocked overnight with or without antibodies. Immunocomplexes were captured for 2 h at room temperature with protein A-agarose beads. Beads were then washed once with wash buffers and twice with Tris-EDTA buffer. Immunoprecipitates were eluted from the beads with 400 μl of elution buffer and digested with 100 μg of proteinase K for 2 h at 42°C and then overnight at 65°C to reverse cross-links. DNA was purified by phenol-chloroform extraction, precipitated by ethanol, and then used as a template for PCRs.
To prepare chromatin DNA from mouse tissues, postnatal day 6 (P6) cerebella were dissected and incubated for 15 min at room temperature in 1% formaldehyde solution. Cross-linking was terminated by the addition of glycine to a final concentration of 0.125 M. Fixed tissues were washed twice with ice-cold PBS buffer, homogenized, and centrifuged at 2,000 rpm (Eppendorf 5415C) for 4 min. After washing of the pellet with ice-cold PBS containing protease inhibitors, nuclei were prepared using the NE-PER nuclear and cytoplasmic extraction kit (Pierce Biotechnology) and resuspended in SDS lysis buffer to prepare chromatin DNA for the ChIP assay as described above. The anti-Barhl1 antibody was described previously (19), and the control anti-RNA polymerase II (Pol II) antibody and IgG were available commercially (Active Motif). The PCR primers flanking the first candidate Barhl1 and Barhl2 binding site (see below) were as follows: 5′AAAACACCCTGAAAATAAAATTTTAAATGCTTC3′ and 5′CGCCTCTAATGAAGATGATTAGCCAAGGGGGGCA3′. Those flanking the second candidate Barhl1 and Barhl2 binding site were as follows: 5′ACCAGAGTGAGGCCTAATTCGGAGGCGAGAGC3′ and 5′CCTTTCTTCCTCCTAATTTCCCTTCCTCATCCC3′.
RESULTS
5′ and 3′ flanking sequences involved in conferring the endogenous Barhl1 expression pattern.
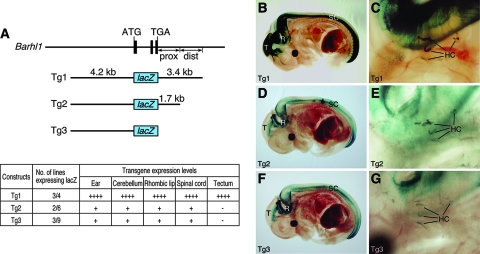
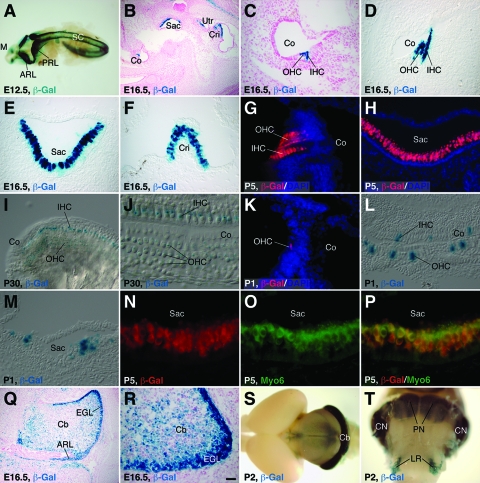
As a first step to define DNA sequences in the Barhl1 gene that can direct specific gene expression to the inner ear and CNS, we constructed a transgene (Tg1) containing approximately 4.2 kb of 5′ flanking and 3.4 kb of 3′ flanking DNA sequences fused to a lacZ reporter (Fig. 1A). Among the four founder lines obtained for this transgenic construct, three lines displayed lacZ expression with similar patterns in the inner ear and CNS (Fig. 1A). From embryonic day 13.5 (E13.5) to early postnatal stages, X-Gal staining and β-Gal immunofluorescence revealed that the transgene was expressed strongly in both inner hair cells and outer hair cells of the organ of Corti, as well as in hair cells of the saccule, utricle, and cristae of the vestibular system (Fig. 1C and 2B to H). Double immunolabeling showed that β-Gal was probably expressed in all hair cells that were immunoreactive with the hair cell-specific marker Myo6 (Fig. 2N to P) (10, 39), indicating that Tg1 was expressed in the inner ear in a pattern that recapitulated that of the endogenous Barhl1 gene (4, 18). Consistent with this notion, there was significant downregulation of Tg1 expression in inner ear hair cells at late postnatal and adult stages (Fig. 2I and J), similar to that of endogenous gene expression (see Fig. S1 in the supplemental material) (18).
FIG. 1.
Expression patterns of the lacZ reporter driven by Barhl1 transgenes. (A) Schematics of the Barhl1 genomic locus, transgenic constructs, and transgene expression levels in various tissues. The black bars represent the three coding exons of Barhl1, and the positions of the initiator codon ATG and the stop codon TGA are indicated. Indicated also are the proximal (prox) and distal (dist) 3′ 1.7-kb fragments. Expression levels were scored as follows: ++++, strong; +, weak; and −, absent. (B to G) X-Gal staining of E13.5 whole-mount embryos containing the indicated transgene followed by clearing in benzyl alcohol-benzyl benzoate. Strong β-Gal activity in the tecta (T), rhombencephala (R), and spinal cords (SC) of Tg1 embryos was seen (B), whereas in Tg2 and Tg3 embryos, only weak activity in the rhombencephala and spinal cords was observed and there was no activity in the tecta (D and F). Additionally, all three transgenes were expressed in the inner ear hair cells (HC) (C, E, and G).
FIG. 2.
β-Gal expression in the inner ear and brain from the Tg1 or Tg3 transgene. (A to J) Using Tg1 animals at the indicated stages, X-Gal staining was performed on whole-mount embryos (A), inner ear sections (B to F), and whole-mount organs of Corti (I and J) or immunofluorescence was carried out on inner ear sections with an anti-β-Gal antibody (G and H). (A) β-Gal activity was seen in the mesencephala, anterior and posterior rhombic lips, and spinal cords at E12.5. (B to J) In the inner ear sections, β-Gal activity was present in hair cells of the cochlear and vestibular systems and this activity was significantly downregulated by P30. (K to M) Inner ear sections of Tg3 neonates were immunolabeled with an anti-β-Gal antibody (K) or stained with X-Gal (L and M). β-Gal activity was observed only in scattered hair cells of the organs of Corti (K and L) and saccules (M). (N to P) Saccular sections from P5 Tg1 animals were labeled by a double-immunofluorescence method with antibodies against β-Gal and Myo6. Note the complete colocalization of β-Gal and Myo6 in hair cells. (Q to T) X-Gal staining of brain sections (Q and R) and whole-mount brains (S and T) from Tg1 animals at the indicated stages. Strong β-Gal activity was seen in the cerebellar granule cells, rhombic lips, PN, CN, and LR nuclei. Sections in panels B, C, Q, and R were counterstained with Fast Red, and those in panels G, H, and K were counterstained with nuclear DAPI. ARL, anterior rhombic lip; Cb, cerebellum; Co, cochlea; Cri, crista; EGL, external granule layer; IHC, inner hair cell; M, mesencephalon; OHC, outer hair cell; PRL, posterior rhombic lip; Sac, saccule; SC, spinal cord; Utr, utricule. Scale bars, 100 μm (B and Q), 25 μm (C to I, K to M, and R), 12.5 μm (J), and 10 μm (N to P).
In the developing CNS, Tg1 was expressed in the diencephalon, mesencephalon, rhombencephalon, and spinal cord, also in patterns similar to those of the endogenous gene (Fig. 1A and B and 2A and Q to T) (4, 19, 20). In particular, Tg1 was prominently expressed by the tectum of the midbrain, the anterior and posterior rhombic lips, the external granule layer of the cerebellum, the dorsal spinal cord, and precerebellar nuclei, including the pontine nucleus (PN), the cochlear nucleus (CN), and the lateral reticular (LR) nucleus (Fig. 1B and 2A and Q to T; also data not shown). Thus, the 4.2-kb 5′ flanking sequence and the 3.4-kb 3′ flanking sequence together were sufficient to recapitulate the spatial and temporal expression patterns of the endogenous Barhl1 gene in the inner ear and CNS.
Hair cell-specific transgene expression driven by a 4.2-kb Barhl1 promoter sequence.
Given the sufficiency of Tg1 to drive strong reporter gene expression in the inner ear and CNS, we next investigated whether its 3′ flanking sequence was necessary for high-level tissue-specific gene expression. Two additional transgenes were constructed from Tg1 by deleting either the distal 1.7-kb 3′ flanking sequence from the 3′ end (Tg2) or the entire 3.4-kb 3′ fragment (Tg3) (Fig. 1A). Tg2 and Tg3 displayed indistinguishable patterns of β-Gal expression in the inner ear and CNS (Fig. 1A and D to G). In the inner ear, both Tg2 and Tg3 directed β-Gal expression specifically to hair cells of both the cochlear and vestibular systems (Fig. 1E and G), suggesting that the 4.2-kb promoter sequence alone was sufficient to confer hair cell-specific expression in the inner ear. However, the β-Gal activities in the inner ears of Tg2 and Tg3 animals were substantially weaker than that in the inner ears of Tg1 mice, and the expression was visible only in scattered hair cells (Fig. 2B to H and K to M; also data not shown), indicating the existence of an enhancer within the distal 1.7-kb 3′ flanking sequence that directs strong Barhl1 gene expression to the inner ear (Fig. 1A).
In the CNSs of Tg2 and Tg3 mice, there was also a dramatic reduction of β-Gal expression compared to that in Tg1 animals (Fig. 1B, D, and F). Moreover, β-Gal expression was completely absent from the tecta of Tg2 and Tg3 embryos, even though it was still present in the rhombencephala and spinal cords (Fig. 1D and F), indicating a change in the tissue specificity of gene expression. Therefore, unlike in the inner ear, in the CNS the distal 1.7-kb 3′ flanking sequence of Barhl1 was required not only for strong expression but also for tissue-specific expression.
Auto- and cross-activation of the Barhl1 promoter by Barhl1 and Barhl2.
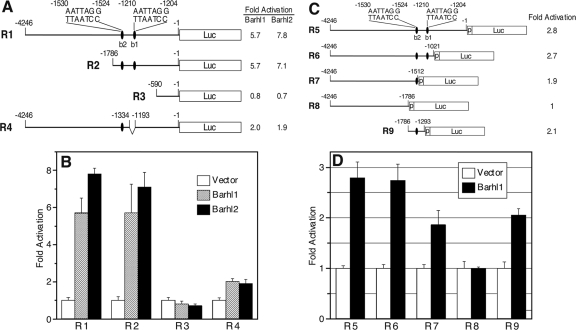
Given the expression of Tg3 in inner ear hair cells and CNS neurons and the expression of Barhl1 and/or Barhl2 in the same cells (4, 18, 19, 26, 31), it is possible that the 4.2-kb Barhl1 promoter sequence may be auto- and cross-regulated by Barhl1 and Barhl2. We tested this possibility by fusing the 4.2-kb promoter sequence to a luciferase reporter gene in the pGL3-Basic vector (R1) and then measuring luciferase activity in transient transfection and transcription assays. The results of these assays revealed that both Barhl1 and Barhl2 could increase the luciferase activity by severalfold in the 293T human embryonic kidney cells and P19 embryonic carcinoma cells (Fig. 3A and B and data not shown). Thus, the 4.2-kb promoter sequence can indeed be auto- and cross-activated by Barhl1 and Barhl2.
FIG. 3.
Transcriptional activities of Barhl1 and Barhl2 on deletion constructs of the 4.2-kb Barhl1 5′ promoter sequence. (A) Schematic of 5′ and internal-deletion luciferase (Luc) reporter constructs (R1 to R4) and their relative activities activated by Barhl1 and Barhl2. All constructs were made with the pGL3-Basic vector. The ovals indicate putative Barhl1 and Barhl2 binding sites (b1 and b2). (B) Levels of activation (n-fold) of the luciferase activities of constructs R1 to R4 by Barhl1 and Barhl2. Each histogram represents the means ± the standard deviations (SD) of results from triplicate assays in a single experiment, and all experiments were repeated three times with similar results. (C) Schematic of 3′ deletion luciferase reporter constructs (R5 to R9) and their relative activities activated by Barhl1. All constructs were made with the pGL3-promoter (p) vector. The ovals indicate putative Barhl1 and Barhl2 binding sites (b1 and b2). (D) Levels of activation of the luciferase activities of constructs R5 to R9 by Barhl1. Each histogram represents the means ± SD of results from triplicate assays in a single experiment, and all experiments were repeated three times with similar results.
We next narrowed down the region that contains Barhl1 and Barhl2 cis-acting elements in the 4.2-kb Barhl1 promoter sequence by making a series of 5′ truncation, 3′ truncation, and internal-deletion luciferase reporter constructs (R2 to R4 and R6 to R9) (Fig. 3A and C). Compared to the luciferase activity of the full-length construct, R1, activated by Barhl1 or Barhl2, the 5′ deletion construct R2 did not display any change of activity but R3 showed a sharp 7- to 10-fold drop in luciferase activity (Fig. 3A and B). Therefore, it appeared that all cis-acting elements necessary for Barhl1 and Barh2 activation were confined to a 1.2-kb region from bp −1786 to −589 (Fig. 3A). A 142-bp internal deletion in this region, from bp −1333 to −1192 (R4), resulted in a three- to fourfold decrease in luciferase activity, indicating the presence of a potential cis-acting element(s) in this short sequence. The findings from 3′ deletion analyses using the pGL3-Promoter vector supported these conclusions, since a deletion from bp −1021 to −1511 resulted in a moderate decrease in luciferase activity activated by Barhl1 and a deletion from bp −1512 to −1785 caused a complete loss of activity (compare data for R6, R7, and R8 in Fig. 3C and D). On the other hand, we found that a 494-bp segment from bp −1786 to −1293 (R9) could largely restore the luciferase activity (Fig. 3C and D), indicating the presence of a potential Barhl1 cis-acting element(s) in this region.
Identification of Barhl1 and Barhl2 binding sites in the Barhl1 promoter.
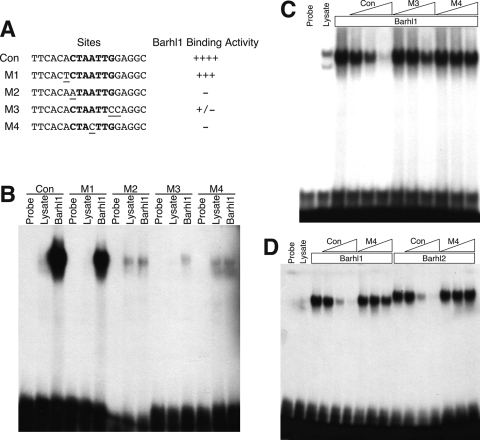
The auto- and cross-activation of the Barhl1 promoter by Barhl1 and Barhl2 homeoproteins suggests the existence of Barh1 and Barhl2 cis-acting elements or binding sites in the promoter region. To identify these sites, we first determined whether Barhl1 and Barhl2 could bind to a consensus homeoprotein binding site, (C/G)TAATTG, that contains the TAAT core motif (5). EMSAs showed strong binding of Barhl1 to the consensus site, whereas mutations in this site, in particular those in the TAAT core motif, greatly diminished or completely abolished the binding activity (Fig. 4A to C). The DNA binding activities of Barhl1 and Barhl2 were specific since their binding to the consensus site could be inhibited by a 500-fold excess of unlabeled consensus sites but was not inhibited by the same amount of cold sites containing a mutation in the TAAT core motif (Fig. 4A, C, and D). Thus, Barhl1 and Barhl2 may bind to CTAATTG or similar homeoprotein binding sites to activate the expression of their target genes.
FIG. 4.
Specific binding of Barhl1 and Barhl2 to a consensus homeoprotein binding site. (A) Summary of the relative DNA binding activity of Barhl1 for the consensus (con) and mutant (M1 to M4) sites. Underlining and boldface indicate mutated bases and those of the consensus homeoprotein binding site, respectively. ++++, strong; +++, intermediate; −, absent; +/−, weak. (B) Results from an EMSA of Barhl1 binding activity for the consensus and mutant sites. The DNA binding activity of in vitro-translated Barhl1 was compared to that of the unprogrammed lysate for each probe. The weak binding with M2 and M4 was nonspecific to Barhl1 since it was equally present in unprogrammed and programmed lysates. (C) EMSAs were performed using the consensus site as the probe in the presence of Barhl1 with increasing concentrations of cold consensus or mutant (M3 or M4) sites. Triangles indicate the progression of molar excesses of cold sites from 10- to 100- to 500-fold. (D) EMSAs were performed using the consensus site as the probe in the presence of Barhl1 or Barhl2 with increasing concentrations of cold consensus or mutant M4 sites. Triangles indicate the progression of molar excesses of cold sites from 10- to 100- to 500-fold.
A sequence search in the 1.2-kb region from bp −1786 to −589, identified above as containing all the potential Barh1 and Barhl2 cis-acting elements, yielded two CCTAATT motifs in reverse orientations between bp −1210 and −1204 (b1) and bp −1530 and −1524 (b2) (Fig. 3A and C). Either or both candidate Barhl1 and Barhl2 binding sites were missing from constructs R3, R4, and R7 to R9, consistent with the observed decrease in luciferase activity (Fig. 3). The b1 site falls into the 142-bp internal deletion of R4, and the b2 site falls into the 494-bp segment of R9 (Fig. 3A and C), in agreement with the suspected presence of Barh1 and Barhl2 cis-acting elements in these two regions. Therefore, Barhl1 and Barhl2 may bind to homeoprotein binding motifs (b1 and b2) to activate transcription from the 4.2-kb Barhl1 promoter sequence.
In vivo binding by Barhl1 to its own promoter.
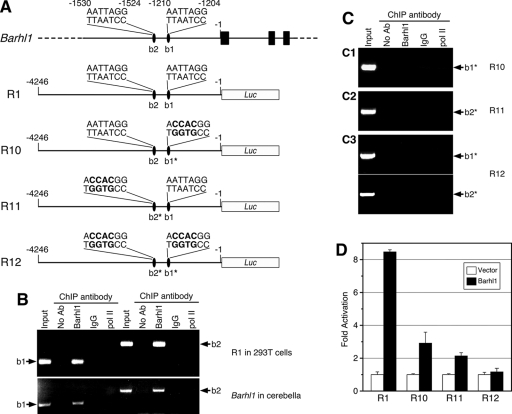
We verified the binding of Barhl1 to the putative homeoprotein binding motifs b1 and b2 by using a ChIP assay (16). Chromatin DNA was prepared from 293T cells cotransfected with the R1 promoter construct and a Barhl1 expression plasmid. An anti-Barhl1 antibody specifically immunoprecipitated promoter fragments that contained b1 or b2 sites, whereas the control anti-RNA Pol II antibody and IgG did not (Fig. 5A and B), demonstrating specific in vivo binding of b1 and b2 sites by Barhl1 in cultured mammalian cells. To determine whether the endogenous Barhl1 promoter could also be bound by Barhl1 at the b1 and b2 sites, we prepared chromatin DNA from P10 mouse cerebella, in which Barhl1 is expressed in granule cells (4, 19). Similar to those in cultured cells, DNA fragments containing b1 or b2 were immunoprecipitated by the Barhl1 antibody but not by control antibodies (Fig. 5B), suggesting that Barhl1 may bind to b1 and b2 sites of its own promoter in vivo to autoactivate the transcription of the endogenous Barhl1 gene.
FIG. 5.
Site-directed mutagenesis of both homeoprotein binding motifs abolishes Barhl1 binding to and autoactivation of the 4.2-kb 5′ promoter sequence. (A) Schematic illustration of the endogenous Barhl1 locus, the wild-type luciferase (Luc) reporter construct (R1) containing the Barhl1 5′ promoter sequence, and the derived single- and double-mutant constructs (R10 to R12). The black bars represent the three coding exons of Barhl1, and the ovals indicate the two putative Barhl1 binding sites (b1 and b2) in the promoter region. As indicated in boldface and with asterisks, the TAAT motif was mutated to GTGG in the b1 and/or b2 binding sites of R10 to R12. (B) Results from a ChIP assay showing in vivo binding of Barhl1 to the b1 and b2 homeoprotein binding motifs in the Barhl1 5′ promoter region. ChIP assays were carried out with chromatin DNA prepared from 293T cells cotransfected with the R1 promoter construct and a Barhl1 expression plasmid (upper panel) or from P10 mouse cerebella (lower panel). Immunoprecipitates were analyzed by PCR using primers flanking the b1 or b2 site. pol II, anti-RNA Pol II antibody; no Ab, no antibody. (C) Results from ChIP assays with chromatin DNA prepared from 293T cells cotransfected with a Barhl1 expression plasmid and the R10 (C1), R11 (C2), or R12 (C3) reporter construct. (D) Levels of activation of the luciferase activities of wild-type and mutant constructs by Barhl1. Each histogram represents the means ± SD of results from triplicate assays in a single experiment, and all experiments were repeated three times with similar results.
Inhibition of Barhl1 binding and autoactivation activities by mutations of both homeoprotein binding motifs in the Barhl1 promoter.
The binding of b1 and b2 sites by Barhl1 does not indicate their necessity for autoregulation, so we investigated whether they are required for this function by site-directed mutagenesis. When either site was mutagenized from CCTAATT to CCGTGGT in the R1 promoter construct to generate mutant constructs R10 and R11 (Fig. 5A), the mutant site lost its Barhl1 binding activity, as determined by ChIP (Fig. 5C). Consequently, Barhl1-induced luciferase activity from R10 and R11 was diminished by three- to fourfold compared to that from the R1 construct (Fig. 5D). When both b1 and b2 sites were simultaneously mutated in the R12 promoter construct (Fig. 5A), the construct lost its Barhl1 binding activity at both b1 and b2 sites and its Barhl1-induced luciferase activity was reduced to the basal level (Fig. 5C and D). Therefore, the presence of both b1 and b2 sites and their binding by Barhl1 appear to be necessary and sufficient for the autoactivation of endogenous Barhl1 gene expression.
Downregulation of Barhl1 expression in Atoh1 null mice.
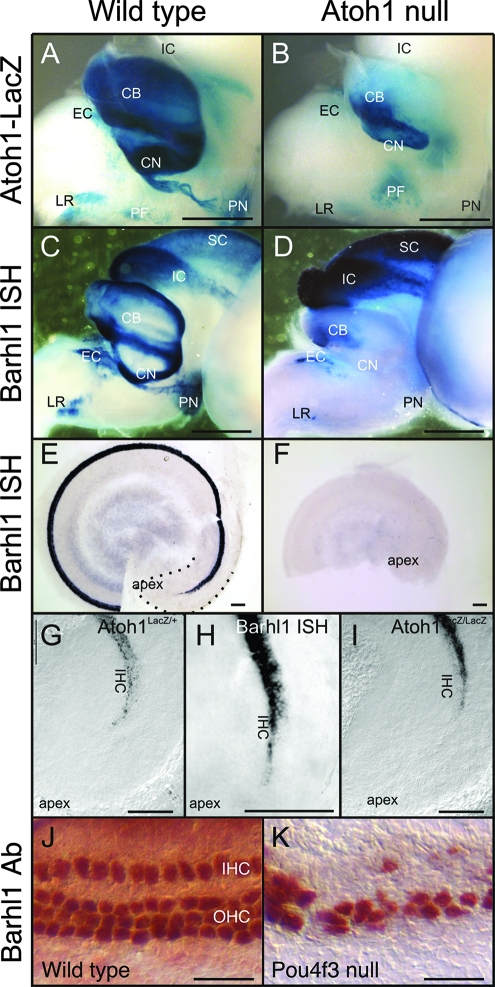
It has been shown previously that the expression of Barhl1 and Barhl2 is under direct and/or indirect regulation by Atoh1 in the spinal cord (2, 31). We therefore investigated the possible regulation of Barhl1 expression by Atoh1 in other CNS areas and the inner ear using Atoh1 null mice (1). In the embryonic mouse, the expression of Barhl1 overlaps only partially with Atoh1 expression. There is little embryonic expression of Atoh1 in the midbrain, and the expression of Barhl1 in the midbrains of Atoh1 null mice was unchanged compared to that in wild-type mice (Fig. 6A to D). In contrast, in areas in which neurons depend on Atoh1 for their differentiation, there was a near complete loss of Barhl1 expression in Atoh1 null animals (Fig. 6D). This was particularly true for the CN and PN, which did not show any expression of Barhl1 in Atoh1 null mice. In other Atoh1-dependent nuclei of Atoh1 null mice, such as the external cuneate (EC) and LR nuclei, as well as in the cerebella, Barhl1 in situ hybridization showed some residual expression up to E18.5. This expression was presumably in postmitotic cells that had not yet degenerated in the absence of Atoh1.
FIG. 6.
Effect of Atoh1 and Pou4f3 inactivation on Barhl1 expression in the ear and brain at E18.5. (A and B) As indicated by the β-Gal activity expressed from the knock-in lacZ reporter, Atoh1 is expressed at high levels in the cerebellum (CB), as well as in the LR nucleus, the PN, the CN, and the EC and perifacial (PF) nuclei (A). In the Atoh1 null brain (B), there is some expression of the lacZ reporter, in particular in the perifacial neurons. However, there is a great loss of neurons and/or lacZ expression in the cerebellum and several nuclei, most notably the PN and CN. IC, inferior colliculus. (C and D) Barhl1 expression in the wild type (C) follows, to some extent, the expression of Atoh1 and is found in the cerebellum and the LR nucleus, PN, and EC nucleus and in some cochlear neurons. Barhl1 is also found in neurons that do not express Atoh1, such as those in the inferior colliculus and the superior colliculus (SC). Some neurons (perifacial nuclei) that are positive for Atoh1 do not show any Barhl1 in situ hybridization (ISH) signals. In the absence of Atoh1 (D), there is a complete loss of Barhl1 expression in the CN and PN and only residual expression in the EC and LR nuclei and the cerebellum. As expected, in areas (interior and superior colliculi) where there is no Atoh1 expression, there is no loss of Barhl1 signals. (E and F) In the ear (E), Barhl1 expression is strong but has not yet reached the cochlear apex at E18.5 (dotted line). Atoh1 null mutant ears (F) show no indication of Barhl1 expression, despite the fact that undifferentiated Atoh1-lacZ-positive cells exist in the apex (I). (G to I) Barhl1 upregulation very closely follows the upregulation of Atoh1, including the absence of expression in the apex and initial upregulation in inner hair cells (IHC) (G and H). In contrast, β-Gal expression in Atoh1 null mice is first detected in the equivalent of outer hair cell (OHC) precursors in the apex (I). (J and K) In the wild-type cochlea, immunostaining with a Barhl1 antibody (Ab) reveals Barhl1 protein expression in the inner and outer hair cells (J). This expression remains strong in residual hair cells of the Pou4f3 null cochlea (K). Scale bars, 1 mm (A to D), 100 μm (E to I), and 25 μm (J and K).
Previous work has demonstrated that Atoh1 is required for the differentiation, but not the initial specification, of cochlear hair cells (1, 9) and that Atoh1 upregulation reaches the apex only around birth. Likewise, the apex was the last part of the cochlea to demonstrate Barhl1 upregulation, and the pattern of this upregulation precisely followed that of Atoh1 (inner hair cells before outer hair cells) (Fig. 6G and H). In contrast, Atoh1-lacZ null mice showed a different pattern of upregulation in the cochlea (outer hair cells before inner hair cells), and no expression whatsoever of Barhl1 in the Atoh1 null cochlea was detected (Fig. 6E, F, and I). These data suggest that Atoh1 may be directly and/or indirectly required for activating and/or maintaining Barhl1 expression in cochlear hair cells and neurons within the CN and PN. The reduced expression of Barhl1 in neurons of the cerebella and EC and LR nuclei of the Atoh1 null mice suggests that these cells can still upregulate Barhl1 in the absence of Atoh1 but are unable to maintain the expression either because they are dying or because they require Atoh1 to maintain the expression. In addition to Atoh1, Pou4f3/Brn3c is required for the maturation and survival of inner ear hair cells (8, 38, 39). In the ears of Pou4f3 null animals, strong Barhl1 protein expression persisted in remaining hair cells (Fig. 6J and K), indicating that Barhl1 expression was unlikely to be regulated by Pou4f3.
DISCUSSION
We have reported previously that Barhl1 is specifically expressed in inner ear hair cells, where it is required for the long-term maintenance of these cells (18), and in developing cerebellar granule cells, precerebellar neurons, and superior collicular neurons, where it is critically involved in migration and survival (19, 20). The experimental data reported here have further defined the Barhl1 regulatory sequences necessary for directing the appropriate spatiotemporal expression pattern of Barhl1. We have found by transgenic analysis that a 4.2-kb 5′ promoter sequence and a 3.4-kb 3′ flanking sequence of the Barhl1 locus together are sufficient to drive high-level reporter gene expression specific to hair cells of the inner ear and sensory neurons within the CNS. Using a deletion-transcription assay, EMSAs, sequence comparisons, ChIP, and site-directed mutagenesis, we have identified two consensus homeoprotein binding motifs within the promoter that can be bound and activated by Barhl1 and Barhl2. By analyzing Atoh1 null mice, we have additionally uncovered a role for Atoh1 in initiating and/or maintaining Barhl1 expression in inner ear hair cells and cerebellar and precerebellar neurons. These studies have allowed us to establish autoregulation as one of the mechanisms underlying the maintenance of Barhl1 cell-specific expression and Atoh1 as a key upstream activator. More importantly, we have identified useful regulatory sequences that can direct strong and specific reporter expression to inner ear hair cells, cerebellar granule cells, and spinal interneurons.
The spatial and temporal expression pattern of Tg1 in the inner ear, cerebellum, precerebellar system, midbrain, and spinal cord mimicked the endogenous Barhl1 pattern previously revealed by RNA in situ hybridization and lacZ knock-in (4, 18-20). Therefore, 4.2-kb 5′ and 3.4-kb 3′ flanking sequences appear to contain all the necessary cis-regulatory elements required for recapitulating the endogenous Barhl1 expression pattern. This information provides a framework for further identifying upstream regulatory factors and for exploring the molecular basis of tissue-specific gene expression. More importantly, the defined regulatory sequences represent very useful reagents for researchers interested in the development and function of inner ear hair cells, cerebellar granule cells, and spinal sensory neurons. In particular, Tg1 perhaps represents one of the best hair cell-specific transgenes thus far available. In the inner ear, it is expressed only and broadly by hair cells of the organ of Corti and vestibular end organs. In contrast, none of the few other hair cell-specific transgenes described to date display hair cell expression in such uniformity, and they usually have a later onset of expression within hair cells (3, 17, 37, 42). For instance, the previously described Myo7a yeast artificial chromosome transgene, the Chrna9 bacterial artificial chromosome transgene (encoding an α9 acetylcholine receptor), and the Prestin bacterial artificial chromosome transgene direct reporter expression to hair cells, but they all show regional specificity within the organ of Corti (3, 37, 42). Other transgenes, including those derived from Atoh1 and Pou4f3, are expressed in but not specific to hair cells (6, 11, 25, 33). Hence, the Barhl1 regulatory sequences defined here would provide additional tools for conditional hair cell-specific gene knockout, the characterization of hair cell-affecting mutants, and the study of properties of deafness gene products (25, 36, 41).
The observation that Tg2 exhibited much weaker expression than Tg1 and lost tectum expression suggests the existence of an enhancer element(s) within the 3′ distal 1.7-kb fragment that directs strong and tectum-specific Barhl1 expression. Interestingly, there is a 3′ enhancer present in the Barhl2 and Atoh1 loci that can be activated by Atoh1 and is critical for high-level reporter gene expression (11, 31). It is tempting to assume that Barhl1 and Barhl2 have similar 3′ enhancers given their similar spatiotemporal expression patterns during neurogenesis. However, a sequence comparison failed to identify any motif similar to the Barhl2 enhancer sequence in the 3′ distal 1.7-kb fragment of Barhl1. This finding may be hardly surprising since the Barhl2 enhancer is specific to the spinal cord (31) whereas the Barhl1 enhancer is required for high-level and tectum-specific expression. Therefore, the similar expression patterns of Barhl1 and Barhl2 may be controlled by a mechanism much more complex than anticipated, involving cis-regulatory elements common to several tissues as well as tissue-specific modular elements.
Reporter gene expression driven by the 4.2-kb 5′ promoter (Tg3) was shown to retain specificity to the inner ear, rhombencephalon, and spinal cord, albeit occurring at a much reduced level compared to that of Tg1. This low level of transgene expression may explain the scattered but nevertheless hair cell-specific expression within the inner ear. Alternatively, the scattered labeling of hair cells may reflect an altered mosaic expression pattern. In either case, the 5′ promoter must harbor cis-acting elements necessary for the low-level and yet tissue-specific expression. We utilized transcriptional, DNA binding, ChIP, and mutational assays to demonstrate that Barhl1 and Barhl2 can auto- and cross-activate gene expression from the 4.2-kb promoter, suggesting that Barhl1 expression may be maintained partly by a positive feedback mechanism in the hair cells and CNS. In Drosophila, misexpressed BarH1 and BarH2 could induce ectopic BarH1 and BarH2 expression in the eye imaginal disk, suggesting that once initiated by secreted signaling molecules, BarH1 and BarH2 expression is maintained by autoactivation in basal undifferentiated cells (22). Therefore, autoregulation appears to be evolutionarily conserved among Drosophila and mammalian BarH homologs, perhaps as a parsimonious mechanism of maintaining their expression.
Besides autoregulation, other parallel mechanisms must exist for the maintenance of Barhl1 expression, as the knocked-in lacZ reporter retains its expression within the hair cells of homozygous mouse mutants (18). Obviously, regulatory factors activating the 3′ enhancer are involved. A sequence analysis of the 3′ distal 1.7-kb fragment revealed the existence of two E boxes (CAGCTG) that can be bound by Atoh1 (11, 31), suggesting Atoh1 as a possible upstream activator. Indeed, our data indicate that Barhl1 is in part regulated by Atoh1 and in part independent of Atoh1. More specifically, we found four different sets of neurons and cells with respect to Atoh1 and Barhl1 interaction: some cells did not show any Atoh1 expression but were positive for Barhl1 (superior and inferior colliculi). At the other extreme, there were cells that expressed Atoh1 but did not express Barhl1 (perifacial neurons). The cells that showed the coexpression of Atoh1 and Barhl1 seemed to fall into two categories. In one case (that of hair cells, CN, and PN), there was a complete loss of Barhl1 expression in Atoh1 null mice, suggesting the necessity of Atoh1 in controlling Barhl1 expression. In the second category (cerebellar cells and EC and LR nuclei), there was some residual upregulation of Barhl1 in Atoh1 null mice, and the loss of expression may reflect either the need for the continued presence of Atoh1 to maintain Barhl1 expression or the fact that these cells die and thus do not show appreciable levels of expression of Barhl1. Most interestingly, cochlear hair cells coexpressed both genes, and these hair cells die according to different time courses in the respective mutants (6, 18). An interesting possibility for future study in this case may be that the differential expression of Barhl1 in hair cells through an Atoh1 promoter may rescue hair cells that are otherwise lost in the Atoh1 null mutant.
In this work, we used a combination of in vivo and in vitro approaches to define two closely located Barhl binding sites, b1 and b2 (∼300 bp apart), within the 4.2-kb 5′ promoter necessary for autoactivation. These two cis-acting elements are functionally and physiologically relevant. First, β-Gal expression driven by the 4.2-kb 5′ promoter was limited to the inner ear, rhombencephalon, and spinal cord, where endogenous Barhl1 and/or Barhl2 are expressed. Second, both b1 and b2 sites (CCTAATT) are AT-rich and closely resemble the canonical homeoprotein binding motif (C/G)TAATTG (5). Third, these sites can be occupied in vivo by Barhl1 not only in cultured cells but also in cerebellar neurons, as demonstrated by the ChIP assay. Finally, site-directed mutagenesis of both binding sites eliminated Barhl1 binding to and activation of the 4.2-kb 5′ promoter. It appears that both b1 and b2 sites are required for the maximal level of expression from the promoter. The mutation of either site greatly reduced the activation activity by Barhl1 but did not eliminate it, thereby suggesting a synergistic effect between these two cis-acting elements on the autoregulation of Barhl1 expression. Interestingly, a survey of the CCTAATT motifs among Barhl1 and Barhl2 orthologs identified one to three such sites within a 4-kb promoter region in each of the zebrafish, rat, mouse, and human Barhl1 and Barhl2 genes (see Fig. S2 in the supplemental material), indicating that these sites may be conserved across vertebrate species for auto- and cross-regulation.
Thus far, accumulated evidence shows that the BarH class of homeoproteins act as both positive and negative transcriptional regulators, depending on different target genes, cell types, and developmental contexts. We show in this study that both Barhl1 and Barhl2 can transactivate the Barhl1 promoter. In the organ of Corti-derived cells, however, Barhl1 was found to act as a transcriptional repressor (35). In a teratocarcinoma cell line, Barhl2 was observed to function either as an activator or as a repressor in regulating the expression of basic helix-loop-helix proneural genes (34). During mouse retinogenesis, Barhl2 acts as an activator of the specification of glycinergic amacrine cells but as a repressor of bipolar and Müller cell differentiation (26). The Barhl2 repressive activity is also required for specifying commissural interneuron identity during spinal cord development (31). Similarly, in Xenopus, proper specification of retinal ganglion cells and neural plate patterning depend on the repressor function of Xbarhl2 (27, 29). In Drosophila, BarH1 and BarH2 are able to activate their own promoters but repress atonal expression during retinal neurogenesis (21, 22). Thus, BarH factors from Drosophila to mammals share a common property as dual functional transcriptional regulators of neurogenesis.
Supplementary Material
Acknowledgments
We thank Huda Zoghbi for providing the Atoh1 knockout mice, Bernd Fritzsch and members of the Xiang laboratory for valuable discussion of the project and thoughtful comments on the manuscript, and two anonymous referees for critical comments on an earlier version of the manuscript.
This work was supported in part by the National Institutes of Health (DC004594, EY012020, and EY015777 to M.X. and DC005590 to Bernd Fritzsch).
Footnotes
Published ahead of print on 22 January 2008.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Bermingham, N. A., B. A. Hassan, S. D. Price, M. A. Vollrath, N. Ben-Arie, R. A. Eatock, H. J. Bellen, A. Lysakowski, and H. Y. Zoghbi. 1999. Math1: an essential gene for the generation of inner ear hair cells. Science 2841837-1841. [DOI] [PubMed] [Google Scholar]
- 2.Bermingham, N. A., B. A. Hassan, V. Y. Wang, M. Fernandez, S. Banfi, H. J. Bellen, B. Fritzsch, and H. Y. Zoghbi. 2001. Proprioceptor pathway development is dependent on Math1. Neuron 30411-422. [DOI] [PubMed] [Google Scholar]
- 3.Boeda, B., D. Weil, and C. Petit. 2001. A specific promoter of the sensory cells of the inner ear defined by transgenesis. Hum. Mol. Genet. 101581-1589. [DOI] [PubMed] [Google Scholar]
- 4.Bulfone, A., E. Menguzzato, V. Broccoli, A. Marchitiello, C. Gattuso, M. Mariani, G. G. Consalez, S. Martinez, A. Ballabio, and S. Banfi. 2000. Barhl1, a gene belonging to a new subfamily of mammalian homeobox genes, is expressed in migrating neurons of the CNS. Hum. Mol. Genet. 91443-1452. [DOI] [PubMed] [Google Scholar]
- 5.Catron, K. M., N. Iler, and C. Abate. 1993. Nucleotides flanking a conserved TAAT core dictate the DNA binding specificity of three murine homeodomain proteins. Mol. Cell. Biol. 132354-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, P., J. E. Johnson, H. Y. Zoghbi, and N. Segil. 2002. The role of Math1 in inner ear development: uncoupling the establishment of the sensory primordium from hair cell fate determination. Development 1292495-2505. [DOI] [PubMed] [Google Scholar]
- 7.Colombo, A., G. Reig, M. Mione, and M. L. Concha. 2006. Zebrafish BarH-like genes define discrete neural domains in the early embryo. Gene Expr. Patterns 6347-352. [DOI] [PubMed] [Google Scholar]
- 8.Erkman, L., R. J. McEvilly, L. Luo, A. K. Ryan, F. Hooshmand, S. M. O'Connell, E. M. Keithley, D. H. Rapaport, A. F. Ryan, and M. G. Rosenfeld. 1996. Role of transcription factors Brn-3.1 and Brn-3.2 in auditory and visual system development. Nature 381603-606. [DOI] [PubMed] [Google Scholar]
- 9.Fritzsch, B., V. A. Matei, D. H. Nichols, N. Bermingham, K. Jones, K. W. Beisel, and V. Y. Wang. 2005. Atoh1 null mice show directed afferent fiber growth to undifferentiated ear sensory epithelia followed by incomplete fiber retention. Dev. Dyn. 233570-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasson, T., P. G. Gillespie, J. A. Garcia, R. B. MacDonald, Y. Zhao, A. G. Yee, M. S. Mooseker, and D. P. Corey. 1997. Unconventional myosins in inner-ear sensory epithelia. J. Cell Biol. 1371287-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helms, A. W., A. L. Abney, N. Ben-Arie, H. Y. Zoghbi, and J. E. Johnson. 2000. Autoregulation and multiple enhancers control Math1 expression in the developing nervous system. Development 1271185-1196. [DOI] [PubMed] [Google Scholar]
- 12.Higashijima, S., T. Kojima, T. Michiue, S. Ishimaru, Y. Emori, and K. Saigo. 1992. Dual Bar homeo box genes of Drosophila required in two photoreceptor cells, R1 and R6, and primary pigment cells for normal eye development. Genes Dev. 650-60. [DOI] [PubMed] [Google Scholar]
- 13.Higashijima, S., T. Michiue, Y. Emori, and K. Saigo. 1992. Subtype determination of Drosophila embryonic external sensory organs by redundant homeo box genes BarH1 and BarH2. Genes Dev. 61005-1018. [DOI] [PubMed] [Google Scholar]
- 14.Hogan, B., F. Costantini, and E. Lacy. 1986. Manipulating the mouse embryo: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 15.Kojima, T., S. Ishimaru, S. Higashijima, E. Takayama, H. Akimaru, M. Sone, Y. Emori, and K. Saigo. 1991. Identification of a different-type homeobox gene, BarH1, possibly causing Bar(B) and Om(1D) mutations in Drosophila. Proc. Natl. Acad. Sci. USA 884343-4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavrrar, J. L., and P. J. Farnham. 2004. The use of transient chromatin immunoprecipitation assays to test models for E2F1-specific transcriptional activation. J. Biol. Chem. 27946343-46349. [DOI] [PubMed] [Google Scholar]
- 17.Li, M., Y. Tian, B. Fritzsch, J. Gao, X. Wu, and J. Zuo. 2004. Inner hair cell Cre-expressing transgenic mouse. Genesis 39173-177. [DOI] [PubMed] [Google Scholar]
- 18.Li, S., S. M. Price, H. Cahill, D. K. Ryugo, M. M. Shen, and M. Xiang. 2002. Hearing loss caused by progressive degeneration of cochlear hair cells in mice deficient for the Barhl1 homeobox gene. Development 1293523-3532. [DOI] [PubMed] [Google Scholar]
- 19.Li, S., F. Qiu, A. Xu, S. M. Price, and M. Xiang. 2004. Barhl1 regulates migration and survival of cerebellar granule cells by controlling expression of the neurotrophin-3 gene. J. Neurosci. 243104-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, S., and M. Xiang. 2006. Barhl1 is required for maintenance of a large population of neurons in the zonal layer of the superior colliculus. Dev. Dyn. 2352260-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim, J., and K. W. Choi. 2003. Bar homeodomain proteins are anti-proneural in the Drosophila eye: transcriptional repression of atonal by Bar prevents ectopic retinal neurogenesis. Development 1305965-5974. [DOI] [PubMed] [Google Scholar]
- 22.Lim, J., and K. W. Choi. 2004. Induction and autoregulation of the anti-proneural gene Bar during retinal neurogenesis in Drosophila. Development 1315573-5580. [DOI] [PubMed] [Google Scholar]
- 23.Liu, W., S. L. Khare, X. Liang, M. A. Peters, X. Liu, C. L. Cepko, and M. Xiang. 2000. All Brn3 genes can promote retinal ganglion cell differentiation in the chick. Development 1273237-3247. [DOI] [PubMed] [Google Scholar]
- 24.Lopes, C., A. L. Delezoide, J. M. Delabar, and M. Rachidi. 2006. BARHL1 homeogene, the human ortholog of the mouse Barhl1 involved in cerebellum development, shows regional and cellular specificities in restricted domains of developing human central nervous system. Biochem. Biophys. Res. Commun. 339296-304. [DOI] [PubMed] [Google Scholar]
- 25.Matei, V., S. Pauley, S. Kaing, D. Rowitch, K. W. Beisel, K. Morris, F. Feng, K. Jones, J. Lee, and B. Fritzsch. 2005. Smaller inner ear sensory epithelia in Neurog1 null mice are related to earlier hair cell cycle exit. Dev. Dyn. 234633-650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mo, Z., S. Li, X. Yang, and M. Xiang. 2004. Role of the Barhl2 homeobox gene in the specification of glycinergic amacrine cells. Development 1311607-1618. [DOI] [PubMed] [Google Scholar]
- 27.Offner, N., N. Duval, M. Jamrich, and B. Durand. 2005. The pro-apoptotic activity of a vertebrate Bar-like homeobox gene plays a key role in patterning the Xenopus neural plate by limiting the number of chordin- and shh-expressing cells. Development 1321807-1818. [DOI] [PubMed] [Google Scholar]
- 28.Patterson, K. D., O. Cleaver, W. V. Gerber, F. G. White, and P. A. Krieg. 2000. Distinct expression patterns for two Xenopus Bar homeobox genes. Dev. Genes Evol. 210140-144. [DOI] [PubMed] [Google Scholar]
- 29.Poggi, L., T. Vottari, G. Barsacchi, J. Wittbrodt, and R. Vignali. 2004. The homeobox gene Xbh1 cooperates with proneural genes to specify ganglion cell fate within the Xenopus neural retina. Development 1312305-2315. [DOI] [PubMed] [Google Scholar]
- 30.Reig, G., M. E. Cabrejos, and M. L. Concha. 2007. Functions of BarH transcription factors during embryonic development. Dev. Biol. 302367-375. [DOI] [PubMed] [Google Scholar]
- 31.Saba, R., J. E. Johnson, and T. Saito. 2005. Commissural neuron identity is specified by a homeodomain protein, Mbh1, that is directly downstream of Math1. Development 1322147-2155. [DOI] [PubMed] [Google Scholar]
- 32.Saba, R., N. Nakatsuji, and T. Saito. 2003. Mammalian BarH1 confers commissural neuron identity on dorsal cells in the spinal cord. J. Neurosci. 231987-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sage, C., M. Huang, M. A. Vollrath, M. C. Brown, P. W. Hinds, D. P. Corey, D. E. Vetter, and Z. Y. Chen. 2006. Essential role of retinoblastoma protein in mammalian hair cell development and hearing. Proc. Natl. Acad. Sci. USA 1037345-7350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saito, T., K. Sawamoto, H. Okano, D. J. Anderson, and K. Mikoshiba. 1998. Mammalian BarH homologue is a potential regulator of neural bHLH genes. Dev. Biol. 199216-225. [DOI] [PubMed] [Google Scholar]
- 35.Sud, R., C. M. Jones, S. Banfi, and S. J. Dawson. 2005. Transcriptional regulation by Barhl1 and Brn-3c in organ-of-Corti-derived cell lines. Brain Res. Mol. Brain Res. 141174-180. [DOI] [PubMed] [Google Scholar]
- 36.Tian, Y., S. James, J. Zuo, B. Fritzsch, and K. W. Beisel. 2006. Conditional and inducible gene recombineering in the mouse inner ear. Brain Res. 1091243-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tian, Y., M. Li, B. Fritzsch, and J. Zuo. 2004. Creation of a transgenic mouse for hair-cell gene targeting by using a modified bacterial artificial chromosome containing Prestin. Dev. Dyn. 231199-203. [DOI] [PubMed] [Google Scholar]
- 38.Xiang, M., L. Gan, D. Li, Z. Y. Chen, L. Zhou, B. W. O'Malley, Jr., W. Klein, and J. Nathans. 1997. Essential role of POU-domain factor Brn-3c in auditory and vestibular hair cell development. Proc. Natl. Acad. Sci. USA 949445-9450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiang, M., W. Q. Gao, T. Hasson, and J. J. Shin. 1998. Requirement for Brn-3c in maturation and survival, but not in fate determination of inner ear hair cells. Development 1253935-3946. [DOI] [PubMed] [Google Scholar]
- 40.Xiang, M., L. Zhou, and J. Nathans. 1996. Similarities and differences among inner retinal neurons revealed by the expression of reporter transgenes controlled by Brn-3a, Brn-3b, and Brn-3c promoter sequences. Vis. Neurosci. 13955-962. [DOI] [PubMed] [Google Scholar]
- 41.Zuo, J. 2002. Transgenic and gene targeting studies of hair cell function in mouse inner ear. J. Neurobiol. 53286-305. [DOI] [PubMed] [Google Scholar]
- 42.Zuo, J., J. Treadaway, T. W. Buckner, and B. Fritzsch. 1999. Visualization of α9 acetylcholine receptor expression in hair cells of transgenic mice containing a modified bacterial artificial chromosome. Proc. Natl. Acad. Sci. USA 9614100-14105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.