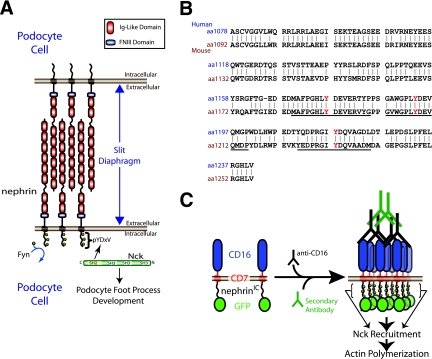
FIG. 1.
(A) Nephrin is a transmembrane protein consisting of eight extracellular immunoglobulin (Ig)-like domains, a fibronectin type three (FNIII) domain, a transmembrane region, and an intracellular region. Nephrin is located at the slit diaphragm, where it interacts with nephrin molecules from adjacent foot processes of podocyte cells. Upon tyrosine phosphorylation by Fyn, nephrin recruits the adaptor protein Nck, which is required for proper foot process formation. (B) An alignment of the intracellular regions of human and mouse nephrin. The tyrosine residues located in the three YDXV motifs are shown (Y is shown in red). The sequences of the mouse peptides used for binding studies are underlined. Numbers to the left of the sequence denote amino acid residues. (C) A schematic of the CD16/7-nephrinIC clustering system is shown. CD16/7-nephrinIC fusion protein localizes to the plasma membrane in cells. Upon the addition of anti-CD16 antibodies to the cellular growth medium, the fusion protein clusters, causing nephrinIC to become phosphorylated and recruit Nck, leading to localized polymerization of actin.