FIG. 7.
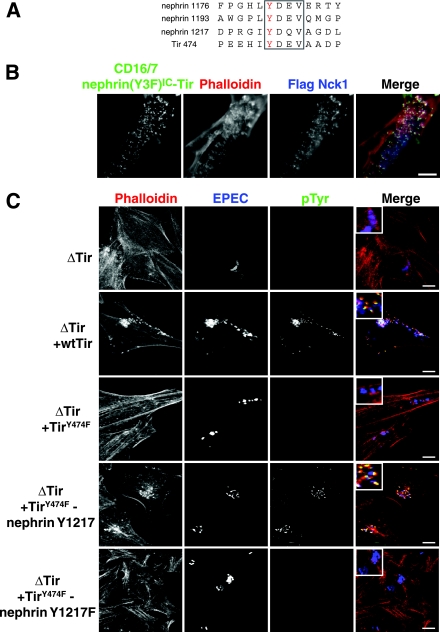
The YDXV motifs of the EPEC Tir protein and nephrin are functionally interchangeable. (A) An alignment of the sequences encompassing the YDXV motifs (boxed) of nephrin and EPEC Tir is shown. The phosphorylated Tyr is colored red. Numbers to the left of the sequence indicate the amino acid numbers of the indicated Tyr residue. (B) Nck-null MEFs were cotransfected with GFP-tagged CD16/7-nephrin(Y3F)IC-Tir and Flag-Nck1 and incubated with anti-CD16 antibodies for 30 min, followed by a 30-min incubation with goat anti-mouse Alexa Fluor 488-conjugated antibodies. Immunofluorescence localization of CD16/7-nephrin(Y3F)IC-Tir (green), Flag-Nck1 (anti-Flag [blue]), and F-actin (phalloidin [red]) is shown. (C) HeLa cells were infected with various strains of EPEC, either without Tir (ΔTir) or with ΔTir reconstituted with WT Tir, TirY474F, TirY474F-nephrin Y1217, or TirY474F-nephrin Y1217F. The EPEC bacteria were visualized with anti-EPEC (blue), sites of tyrosine phosphorylation were visualized with anti-pTyr antibodies (green), and polymerized actin was visualized with phalloidin (red). Bacteria lacking Tir (ΔTir) are impaired in their abilities to adhere to host cells and, thus, show reduced levels of staining for EPEC. Scale bars = 10 μm.