FIG. 3.
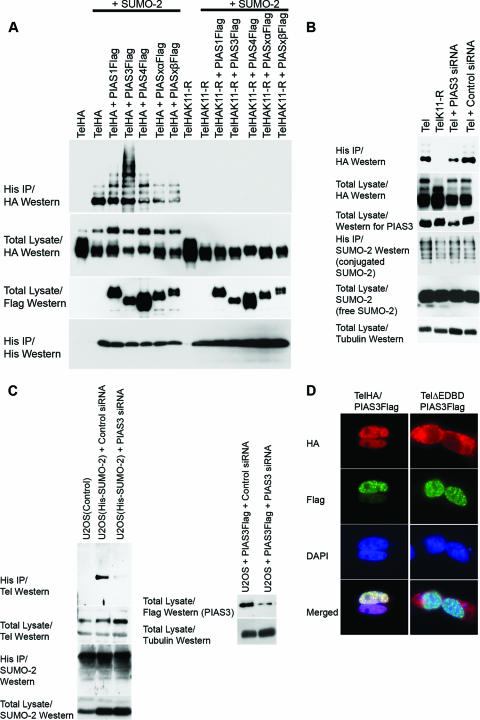
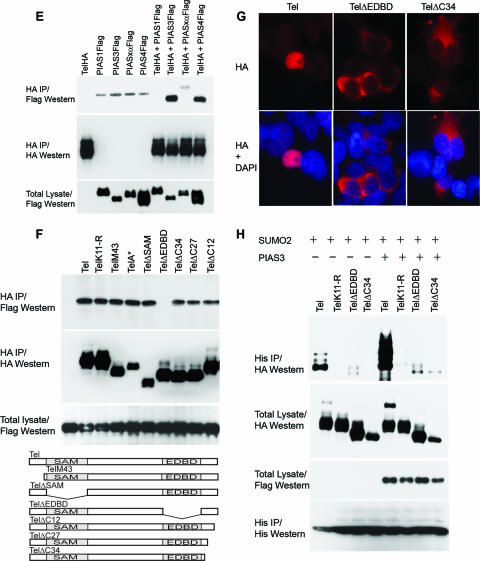
PIAS3 stimulates sumoylation of Tel on K11. (A) Ectopic expression of PIAS3 strongly stimulates sumoylation of K11 of Tel. Tel or TelK11-R was transfected into 293T cells with or without His epitope-tagged SUMO-2, either alone or together with the indicated PIAS constructs. Sumoylated Tel was recovered from cells by a sumoylation assay. (B) Endogenous PIAS3 is essential for normal Tel sumoylation. Cells were transfected with the indicated constructs and a sumoylation assay was performed 2 days later. In the absence of an effective antibody specific for human PIAS3, the efficiency of PIAS3 knockdown was assessed by targeting Flag epitope-tagged PIAS3 expressed in U2OS cells. A nonspecific siRNA was used as a control. (C) Endogenous PIAS3 is required for sumoylation of endogenous Tel. We established U2OS cell lines stably expressing either His epitope-tagged SUMO-1 or SUMO-2. The indicated cell lines were transfected with either a control siRNA (directed against GFP) or siRNAs directed against PIAS3, and a sumoylation assay was performed 2 days later. The efficiency of PIAS3 knockdown was assessed by targeting Flag epitope-tagged PIAS3 expressed in U2OS cells. A nonspecific siRNA was used as a control (right panel). (D) Colocalization of Tel and PIAS3 in the nucleus. Cells were transfected with the indicated constructs and proteins were detected with the indicated antibodies. TelΔEDBD is the same as wild-type Tel except it lacks the EDBD. (E) Tel interacts with PIAS3. 293T cells were transfected with the indicated constructs. Tel complexes were immunopurified from cell lysates, made using radioimmunoprecipitation assay-SDS lysis buffer, using an antibody directed against the HA epitope, and associated PIAS protein was detected using an antibody directed against the Flag epitope. (F) Tel binding to PIAS3 requires its EDBD in cells. The indicated HA epitope-tagged Tel proteins were coexpressed with or without PIAS3 (not shown since the background was clear). Tel complexes were immunopurified from cell lysates using an antibody directed against the HA epitope, and associated PIAS3 protein was detected using an antibody directed against the Flag epitope. Tel M43 lacks the N-terminal 42 amino acids; Tel A* contains an amino substitution (arginine residue in place of an alanine residue) at position 93; TelΔSAM lacks the SAM domain; and Tel ΔC34, TelΔC27, and TelΔC12 have deletions of the C terminus of the indicated lengths. A schematic representation of the mutants tested is included. (G) Mutations that disrupt the EDBD or the C terminus of Tel lead to mislocalization of Tel to the cytoplasm. Cells were transfected with the indicated constructs, and immunohistochemistry was performed with the antibodies shown. (H) Mislocalization strongly abrogates sumoylation. Cells were cotransfected with the indicated constructs, and a sumoylation assay was performed. DAPI, 4′,6′-diamidino-2-phenylindole; IP, immunoprecipitation.