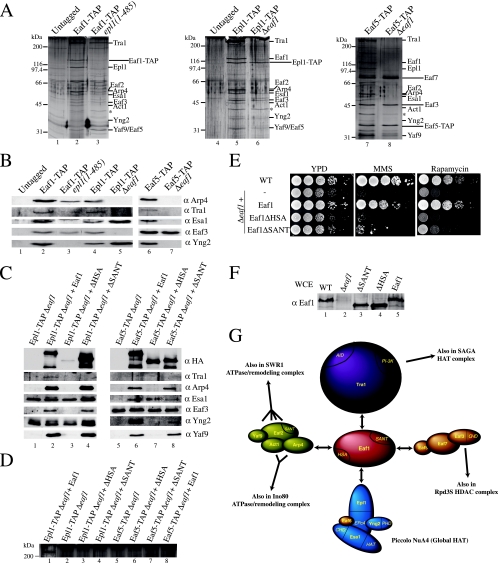
FIG. 3.
Eaf1 serves as a platform for the assembly of four different functional modules into the NuA4 complex. (A) Epl1 interacts with Eaf1 and bridges picNuA4 with the remaining complex, while the association with an Eaf5/7/3 trimer also is dependent on Eaf1. Purified material from untagged strains and the indicated TAP-tagged strains was loaded onto gradient gels and visualized by silver staining. The asterisk corresponds to a nonspecific band known to purify with TAP-tagged proteins. (B) Western blots using the final elution of the TAP purifications shown in panel A were probed with antibodies α against the indicated proteins. (C) The HSA domain region of Eaf1 interacts with the subunits shared with the SWR1/INO80 ATP-dependent chromatin remodeling complexes. Purified material from the indicated TAP-tagged strains supplemented with an episomal version of either EAF1, Eaf1ΔHAS, or Eaf1ΔSANT was loaded onto a gradient gel and analyzed by Western blotting using the antibodies indicated on the right. In lane 7, there is no Esa1 signal but rather a nonspecific keratin band (asterisk). (D) Tra1 requires the SANT domain region of Eaf1 for its association with NuA4. Purified material from strains indicated in the legend to panel C was loaded onto low-percentage gels and visualized by silver staining. The 400-kDa band visible on this part of the gel represents Tra1. (E) Requirement of the HSA and SANT regions of Eaf1 for proper growth. Tenfold dilutions of mid-log-phase Δeaf1, EAF1, Eaf1ΔHSA, and Eaf1ΔSANT yeast cultures were grown in the presence or absence of 0.03% MMS or 25 nM rapamycin. (F) Expression levels of episomal wild-type (WT) and mutant Eaf1 are similar to that of endogenous Eaf1. Whole-cell extracts (WCE) from the strains used for panel E were analyzed by Western blotting with anti-Eaf1. (G) Schematic representation of Eaf1 as the platform for the assembly of the different functional modules of NuA4 and the subunits shared with other chromatin modifying/remodeling complexes.