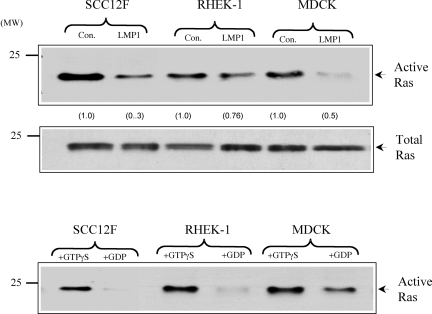
FIG. 3.
LMP1 does not stimulate endogenous c-Ras activity. (A) SCC12F, Rhek-1, and MDCK cells or their LMP1-expressing counterparts were cultured in medium containing 0.5% FCS for 24 h prior to analysis. Equal amounts of total cell lysates (300 μg) were subjected to immunoprecipitation with a GST fusion protein containing the Ras binding domain of c-Raf. The levels of active GTP-bound Ras were determined after immunoblotting of the immunoprecipitates with a monoclonal antibody to Ras (upper panel). The increases (n-fold) in c-Ras activity are indicated in parentheses. Immunoblotting of total cell lysates from control (con.) and LMP1-expressing cells with an antibody to Ras confirmed the presence of equal amounts of endogenous Ras in control and LMP1-expressing cells (middle panel). As an internal assay control, lysates generated from control SCC12F, Rhek-1, and MDCK cells were treated with GTPγS or a 100-fold excess of GDP to activate or inhibit endogenous Ras activity, respectively (lower panel). Numbers on the left of the blots are molecular weight markers.