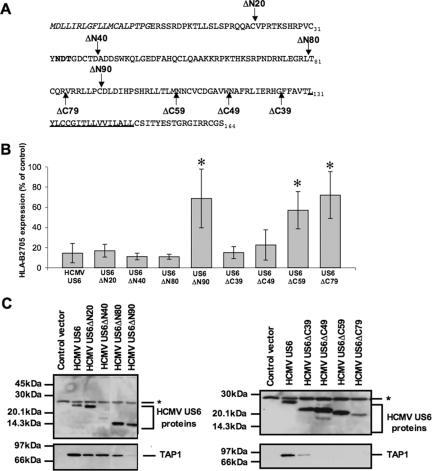
FIG. 2.
Distinct regions of HCMV US6 are required for TAP binding and inhibition. (A) HCMV US6 deletion constructs. The N-terminal signal sequence of HCMV US6 is shown in italic type. Residue 1 corresponds to the N terminus after signal sequence cleavage. The N-linked glycosylation site is shown in bold type, and the predicted transmembrane domain is underlined. Arrows indicate the positions of the N- and C-terminal truncations. (B) Analysis of the effect of HCMV US6 N-terminal and C-terminal truncations on the cell surface expression of HLA-B2705. HeLa-M cells were either transfected with a control vector or cotransfected with a control vector and the HLA-B2705 expression construct or cotransfected with HLA-B2705 and each HCMV US6 truncation. Cell surface expression of HLA-B2705 in the transfectants was quantified by flow cytometry. The level of HLA-B2705 expression is expressed as a percentage of the MFV of the positive control (cells transfected with HLA-B2705 and control vector) after subtracting the MFV of the negative control (cells transfected with control vector only). Each bar represents the level (mean ± standard deviation [error bar]) of HLA-B2705 expression calculated from four independent experiments. Statistical analysis was performed using the Mann-Whitney U test. Those samples with a significantly increased level (P ≤ 0.05) of cell surface HLA-B2705 expression compared to that in cells cotransfected with the HCMV US6 and HLA-B2705 vectors are indicated by an asterisk. (C) Analysis of the interaction between HCMV US6 truncations and TAP. HeLa-M cells were transfected with the control vector, full-length HCMV US6, or each HCMV US6 deletion construct. US6 proteins were immunoprecipitated with the anti-FLAG epitope tag antibody M2 and resolved by SDS-PAGE, transferred onto PVDF membranes, and probed with either TAP1-specific or M2 antibodies. The position of a background band present in all samples that may correspond to the light chain of the M2 antibody is indicated by an asterisk.