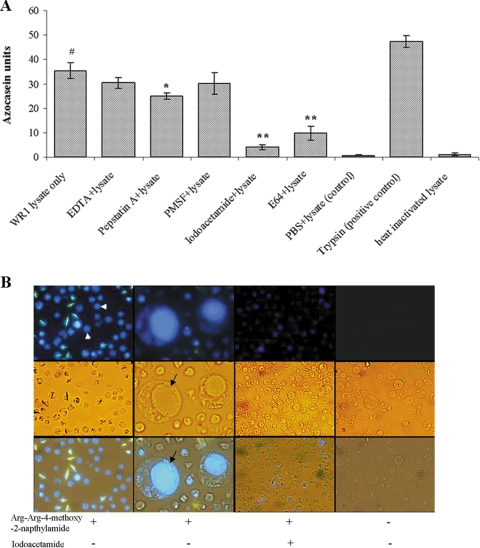
FIG. 1.
Protease activity of B. ratti WR1 and cellular localization of cysteine proteases. (A) Protease activity was determined with azocasein as a substrate. Lysates from 4 × 106 parasites were used for each sample. The assay was performed with and without protease inhibitors as described in Materials and Methods. Significant inhibition of protease activity could be noticed with cysteine protease inhibitors (iodoacetamide and E-64), and less significant inhibition was seen with the aspartic inhibitor pepstatin A. The metalloprotease inhibitor EDTA and the serine inhibitor PMSF showed no inhibition. One azocasein unit was defined as the amount of enzyme producing an increase of 0.01 optical density units/h. The values are means ± standard deviations (n = 3). #, P < 0.01 versus the negative control; *, P = 0.058 versus lysate; **, P < 0.01 versus lysate. (B) Representative fluorescence, light, and merged micrographs show the activities and localization of cysteine proteases in live parasites. Parasites were incubated in a cysteine protease substrate, Arg-Arg-4-methoxy-2-naphtylamide, as described in Materials and Methods. All parasites showed fluorescence (arrowhead, first column on the left), and the higher-magnification (×400) images in the second column show that fluorescence was limited to the central vacuole (arrow) of the parasite. Treatment of parasites with the cysteine inhibitor iodoacetamide resulted in diminished fluorescence (third column). Control parasites without cysteine protease substrate showed no fluorescence (fourth column). (Magnification in first, third, and fourth columns, ×100).