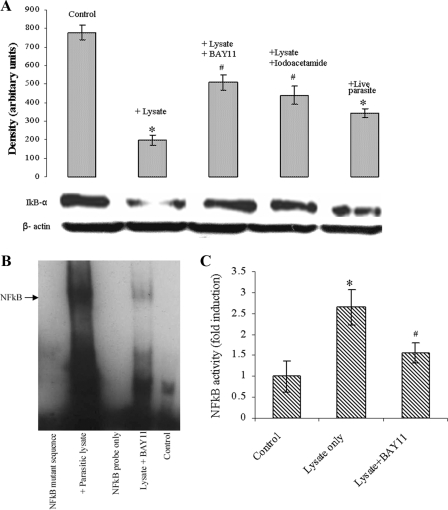
FIG. 5.
B. ratti WR1 exposure to intestinal epithelial cells causes IκB-α degradation and induces NF-κB activation. (A) Live WR1 parasite or lysate coincubation with T84 cells resulted in IκB-α degradation at 5 h, as shown in a representative result with a β-actin loading conrol. The cell lysates were analyzed by Western blotting using an anti-IκB-α antibody. Pretreatment of the cells with the NF-κB inhibitor BAY11-7082 resulted in decreased IκB-α degradation. Iodoacetamide, a cysteine protease inhibitor, decreased the WR1 lysate-induced degradation of IκB-α. The histogram represents densitometry values expressed as arbitrary units ± standard deviations (SD). (B) A representative EMSA shows NF-κB/IL-8 promoter binding activity in nuclear extracts of T84 cells coincubated with WR1 lysate for 6 h. No NF-κB/DNA binding was observed in a mutant NF-κB oligoduplex incubated with nuclear extract, a wild-type NF-κB oligoduplex without nuclear extract, and the control. Pretreatment of T84 cells with the NF-κB inhibitor BAY11-7082 resulted in decreased WR1-induced NF-κB/DNA binding. (C) Histogram showing the increase in NF-κB activity in nuclear extracts of T84 cells. The cells were coincubated with WR1 lysate for 6 h, and NF-κB activity was measured by specific ELISA. The values are means ± SD (n = 3). *, P < 0.05 versus the control; #, P < 0.05 versus WR1 lysate.