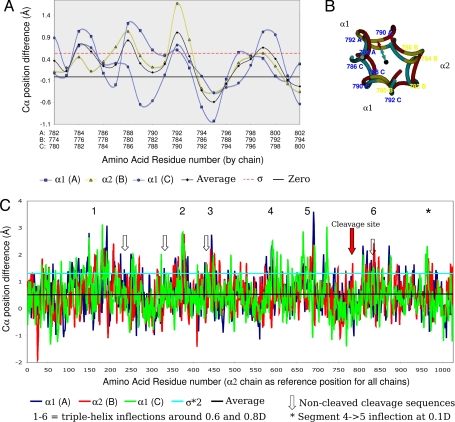
Fig. 2.
Disassociation of peptide chains: difference between the relaxed and stringent models of the collagen triple helix. Sequence numbering includes the N-telopeptide. A and C show the difference of the “from helix center” distances, a measure of triple-helix dissociation of the peptides. The magnitude of dissociation of the three peptide chains, A (α1), B (α2), and C (α1), are shown, along with the average, standard deviation (σ), two times standard deviation (2*σ), or zero, as indicated. (A) The cleavage site region (flanked by 10-aa residues N- and C-terminal). (B) End-on view of central section of cleavage site region. The black dot represents the triple-helix center, the cyan line is the radius of stringent model at 791A Cα, and the red line is the radius of the relaxed model 791A Cα. The difference between the two radiuses is used as the measure of native collagen's triple-helix disassociation (shown as graphs in A and C). (C) As A, except the entire triple helix.