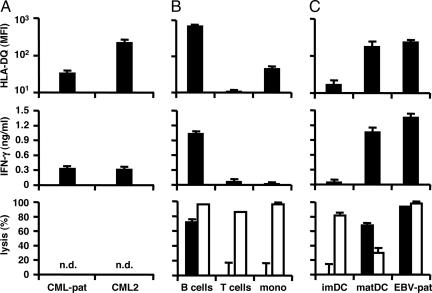
Fig. 4.
Recognition and lysis of hematopoietic cells by LB-PI4K2B-1S-specific CD4+ T cells. (A) CD34+ CML cells isolated from bone marrow mononuclear cells from patient RZ (CML-pat) upon relapse of the malignancy after alloSCT and another HLA-DQB1*0603+ and LB-PI4K2B-1S+ patient with chronic CML (>75% purity, CML2). (B) B cells, T cells, and monocytes isolated from PBMCs from an HLA-DQB1*0603+ and LB-PI4K2B-1S+ healthy individual (>90% purity). (C) Immature and mature DCs and EBV-LCLs from patient RZ were stained with PE-labeled antibodies against HLA-DQ (Leinco Technologies) and analyzed by flow cytometry. (Top) The mean fluorescence intensity (MFI) of two stainings. These cell populations also were used as stimulator cells (3 × 104 cells per well) for CD4+ T cell clone ZRZ38 (5 × 103 cells per well) in IFN-γ ELISA. (Middle) The mean release of IFN-γ (ng/ml) in 50-μl supernatants of triplicate wells. In addition, these cell populations were stained with CFSE and used as target cells (1 × 104 cells per well) for CD4+ T cell clone ZRZ38 and alloreactive HLA-A*0201-specific CD8+ T cell clone MBM13 in CFSE-based cytotoxicity assays at an E/T ratio of 10:1. Numbers of isolated CD34+ CML cells were not sufficient to measure specific lysis. (Bottom) The mean percentage of lysis of CFSE+ cells of triplicate wells after coincubation with clone ZRZ38 (filled bars) and control anti-HLA-A*0201 CD8+ T cell clone MBM13 (open bars). n.d., not done.