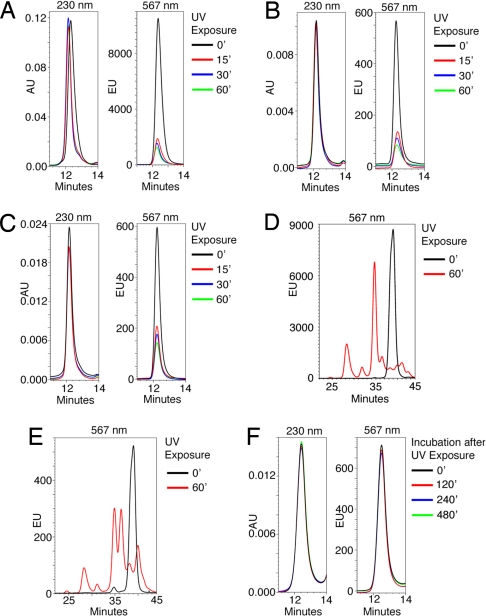
Fig. 2.
UV-induced peptide cleavage and exchange kinetics. (A) HLA-A2-monomers refolded with the fluorescent UV-sensitive peptide Flp*A2 were treated with UV in the presence of the HLA-A2 ligand NLVPMVATV for different time periods and analyzed by gel-filtration HPLC. Absorption at 230 nm (Left) and fluorescence at 567 nm (Right) was measured. (B and C) HLA B7-monomers refolded with either the fluorescent UV-sensitive peptides AARC(Fl)JTLAM (B) and AARGJTLC(Fl)M (C) were treated with UV in the presence of the HLA-B7 ligand TPRVTGGGAM for different time periods and analyzed as in A. (D) HLA-A2-monomers refolded with the fluorescent UV-sensitive peptide Flp*A2 were either left untreated or exposed to UV for 60 min. Extracted peptides were analyzed by reverse-phase HPLC. Black line, untreated; red line, UV-treated. (E) As in D except that before peptide extration, elution material with the retention time of pMHC molecules was isolated by gel-filtration HPLC. Black line, untreated; red line, UV-treated. (F) p*A2-monomers were treated with UV for 30 min in the presence of 0.5 μM fluorescent FLPSDC(FL)FPSV and 49.5 μM FLPSDCFPSV peptide and kept at temperature for the indicated periods before analysis as in A.