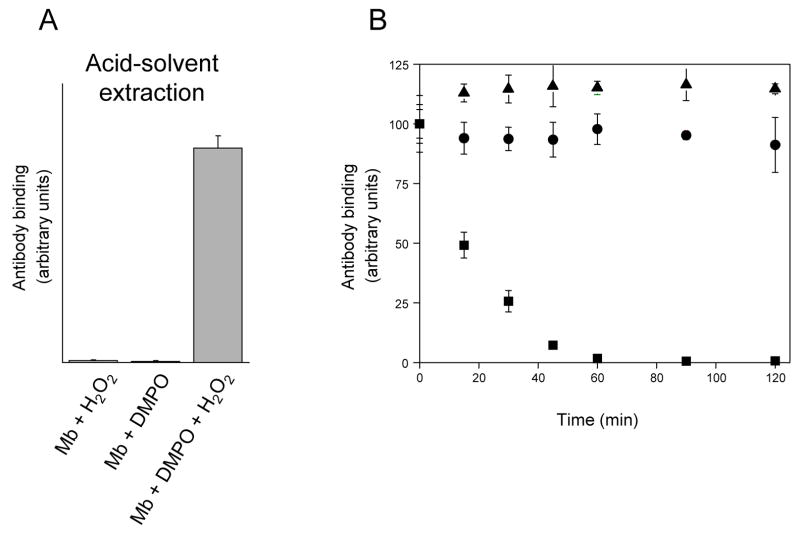
Figure 3.
(A), detection of horse heart myoglobin radical-derived nitrone adducts by immuno-spin trapping: effect of acid-solvent extraction. Reaction mixtures contained horse heart myoglobin (500 μM), DMPO (100 mM) and H2O2 (500 μM), or subsets of these as indicated. The reactions were initiated by adding H2O2 to the mixture of myoglobin and the spin trap. After 10 min incubation at 25 °C, the heme was removed from the globin by acid-solvent extraction as described under Materials and Methods. At the conclusion of the solvent extraction process, reactions were diluted with coating buffer for subsequent ELISA analysis. Values are mean ± S.D (n = 3). (B), effect of trifluoroacetic acid on nitrone adduct stability: time course of adduct decay. DMPO-modified peptides were purified from the tryptic digest of hemoglobin, lyophilized and resuspended in H2O + 0.5% TFA. Excess NH4HCO3 was then added at various times after initiation of the reaction to stop further reaction. The zero time estimates refer to control experiments in which excess NH4HCO3 was added before TFA. At the conclusion of the experiments, reactions were diluted with deionized H2O for ELISA analysis. ▲, DMPO- cysteinyl adduct (peak 1 in Fig. 4); ■, DMPO-tyrosyl adduct (peak 4 in Fig. 4); ●, DMPO-histidinyl adduct (peak 6 in Fig. 4). Values are mean ± S.D (n = 3).