Figure 1.
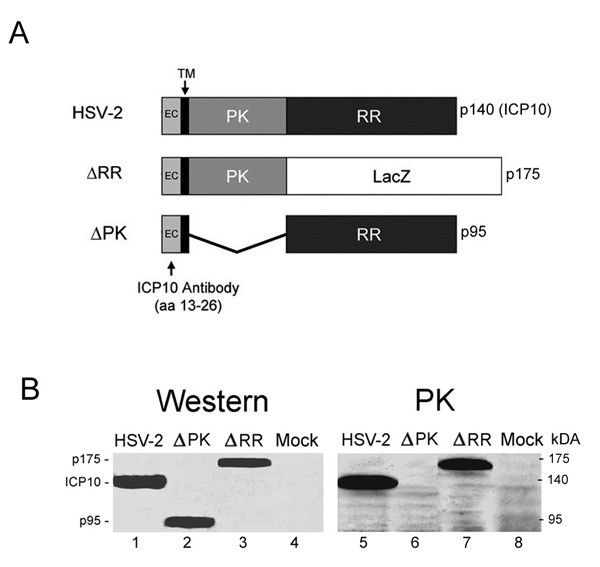
Expression and kinase activity of mutant ICP10 proteins. (A). Schematic representation of the ICP10 and mutant proteins. The wild type ICP10 protein expressed by HSV-2 is a 140 kDa chimera that contains an amino-terminal PK domain and a carboxy-terminal RR domain. In ΔRR, the RR domain was replaced with the β-galactosidase gene (LacZ) which was fused in frame to the PK domain, giving rise to a 175 kDa protein (p175). In ΔPK, the PK domain of ICP10 was deleted giving rise to a 95 kDa protein (p95). All three proteins (ICP10, p175 and p95) retain the transmembrane (TM) and extracellular (EC) domains and amino acids 13–26, which are recognized by the ICP10 antibody. (B). SK-NSH cells infected with HSV-2, ΔRR, ΔPK or PBS (mock-infected) were collected at 18 hrs after infection and cell extracts were assayed for protein expression (western) and ICP10 kinase activity (PK) using immunoblotting and immunocomplex kinase assays with ICP10 antibody.
