Figure 10. Model simulation of the observed kinetic behaviour of Kir6.2wt, Kir6.2ΔN2−30, Kir6.2K185Q, and Kir6.2ΔN2−30/K185Q mutant channels.
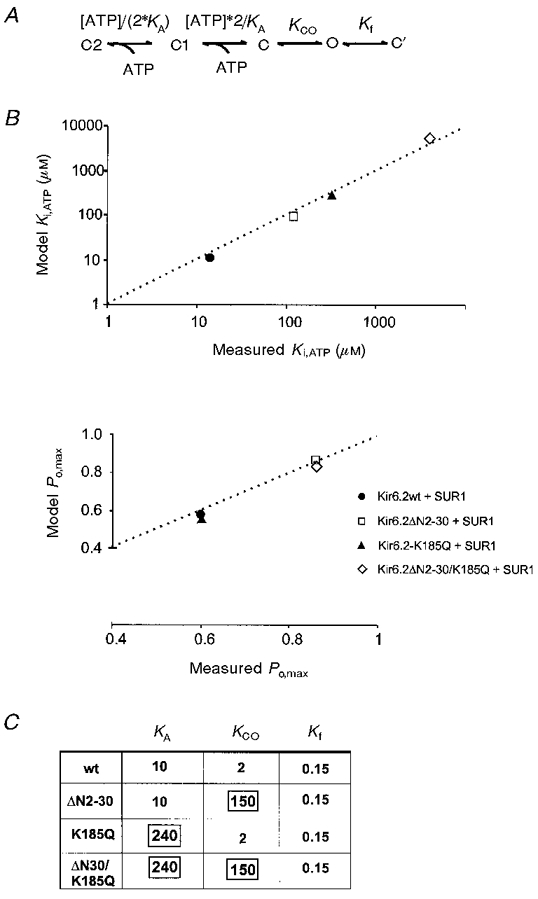
A, model scheme for simulation of KATP channel activity (see Shyng et al. 1997a). Only two sequential ATP binding steps are necessary to approximate the steepness of steady-state dependence of channel activity on [ATP]. C’ refers to the rapid closed state observed within a burst. Equilibrium constants for each transition are indicated. B, model predicted Ki[ATP] and Po,max plotted against measured Ki[ATP] and Po,max, respectively (see Fig. 6A and B. C), table showing the model parameters for equilibrium constants (KCO and Kf) and the ATP binding constant (KA). Only the boxed constants were altered to produce the necessary simulations of mutant channel activity.
