Figure 10. A hypothetical schematic model of channel gating kinetics of the NSC channel for untreated (A) and terbutaline-treated (B) conditions.
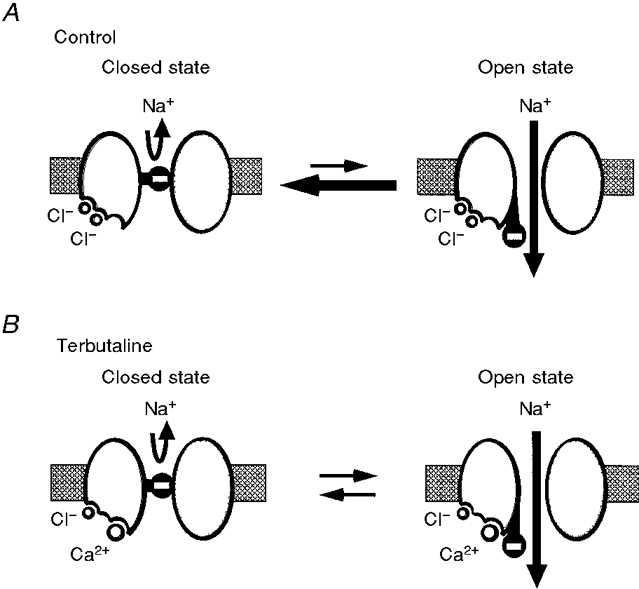
The channel has two Cl−-binding sites and one Ca2+-binding site which regulate the channel activity. A, the cytosolic Cl−-binding sites are almost always occupied by Cl− in untreated channels. The cytosolic Ca2+-binding site is almost never occupied by Ca2+. If the channel gate has a negative charge, two Cl− ions, with their negative charges, bound to the channel promote channel closing. B, on average, the two binding sites for cytosolic Cl− are usually occupied by cytosolic Cl− for only about half the time in terbutaline-treated channels, i.e. on average only one of the two binding sites for cytosolic Cl− is occupied by cytosolic Cl− in terbutaline-treated channels. The cytosolic Ca2+-binding site is almost always occupied by Ca2+. In the terbutaline-stimulated case, one Cl− ion and one Ca2+ ion, with one net positive charge, bound to the channel cause the channel to remain in the open state for a longer time, leading to a decrease in the closing rate.
