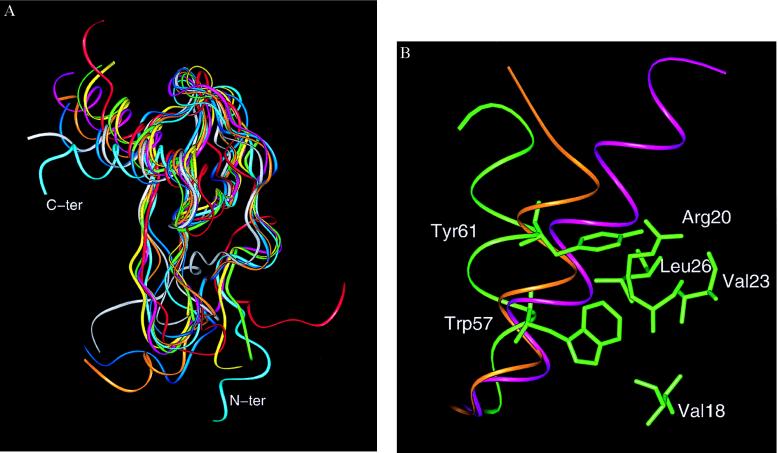
Figure 3.
(A) Diagram of the superimposed monomers of SDF-1α (cyan), gro-α/melanoma growth-stimulating activity (red), neutrophil-activating peptide 2 (yellow), platelet factor 4 (green), IL-8 (pink), RANTES (white), monocyte chemoattractant protein 1 (ochre), and MIP-1β (blue), highlighting differences at the N-terminal region and the C-terminal helix. (Figure generated with the graphics program insightii). The Cα superposition was based on lsqman (16) with an initial framework of Cys-9, Cys-34, Cys-50, and Trp-57 in SDF-1α and their equivalent counterparts in the other chemokines. The angle of the helix with respect to the β-sheet was measured by using the Cα from the three residues 49 (from β3), 54 (from the beginning of the helix), and 66 (from the end of the helix) in SDF-1α. The equivalent residues were used for measuring the angles in the other chemokine structures. (B) Packing of the C-terminal helix and relevant side chains in SDF-1α (green). The ribbon diagram of the α-helix of IL-8 (purple) and RANTES (orange) is shown for reference. The van der Waals interactions involving Tyr-61 are believed to be pivotal in the “open” orientation of the helix of SDF-1α.