Figure 15. Effects of hyposmotic shock and tyrphostin A23 (TY23) on phosphorylation of tyrosine.
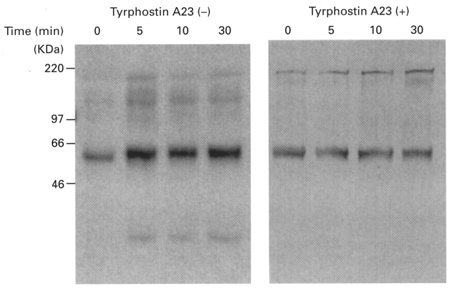
After exposure of cells to a hypotonic solution with or without pretreatment of tyrphostin A23 (100 μM, bilateral application, 1 h), cell lysates were prepared. The cell lysates containing 25 μg protein were subjected to 10 % SDS-PAGE and then immunoblotted with anti-phosphotyrosine antibody, PY99. Phosphorylation of tyrosine increased with time after cells had been exposed to a hyposmotic solution. Tyrosine of 60 kDa proteins was mainly phosphorylated by hyposmotic shock. Tyrphostin A23 (TY23, 100 μM) blocked the hyposmolality-induced phosphorylation of tyrosine.
