Abstract
The present study examined whether reductions in muscle blood flow with exercise-induced dehydration would reduce substrate delivery and metabolite and heat removal to and from active skeletal muscles during prolonged exercise in the heat. A second aim was to examine the effects of dehydration on fuel utilisation across the exercising leg and identify factors related to fatigue.
Seven cyclists performed two cycle ergometer exercise trials in the heat (35°C; 61 ± 2% of maximal oxygen consumption rate, VO2,max), separated by 1 week. During the first trial (dehydration, DE), they cycled until volitional exhaustion (135 ± 4 min, mean ±s.e.m.), while developing progressive DE and hyperthermia (3.9 ± 0.3% body weight loss and 39.7 ± 0.2°C oesophageal temperature, Toes). On the second trial (control), they cycled for the same period of time maintaining euhydration by ingesting fluids and stabilising Toes at 38.2 ± 0.1°C.
After 20 min of exercise in both trials, leg blood flow (LBF) and leg exchange of lactate, glucose, free fatty acids (FFA) and glycerol were similar. During the 20 to 135 ± 4 min period of exercise, LBF declined significantly in DE but tended to increase in control. Therefore, after 120 and 135 ± 4 min of DE, LBF was 0.6 ± 0.2 and 1.0 ± 0.3 l min−1 lower (P < 0.05), respectively, compared with control.
The lower LBF after 2 h in DE did not alter glucose or FFA delivery compared with control. However, DE resulted in lower (P < 0.05) net FFA uptake and higher (P < 0.05) muscle glycogen utilisation (45%), muscle lactate accumulation (4.6-fold) and net lactate release (52%), without altering net glycerol release or net glucose uptake.
In both trials, the mean convective heat transfer from the exercising legs to the body core ranged from 6.3 ± 1.7 to 7.2 ± 1.3 kJ min−1, thereby accounting for 35-40 % of the estimated rate of heat production (∼18 kJ min−1).
At exhaustion in DE, blood lactate values were low whereas blood glucose and muscle glycogen levels were still high. Exhaustion coincided with high body temperature (∼40°C).
In conclusion, the present results demonstrate that reductions in exercising muscle blood flow with dehydration do not impair either the delivery of glucose and FFA or the removal of lactate during moderately intense prolonged exercise in the heat. However, dehydration during exercise in the heat elevates carbohydrate oxidation and lactate production. A major finding is that more than one-half of the metabolic heat liberated in the contracting leg muscles is dissipated directly to the surrounding environment. The present results indicate that hyperthermia, rather than altered metabolism, is the main factor underlying the early fatigue with dehydration during prolonged exercise in the heat.
Dehydration during prolonged exercise in the heat is known to exacerbate cardiovascular strain, increase core temperature and impair endurance performance compared with when fluids are ingested during exercise (Pitts et al. 1944; Saltin, 1964; Sawka et al. 1979; Nielsen et al. 1981; Hamilton et al. 1991; Montain & Coyle, 1992a,b; González-Alonso et al. 1995, 1997, 1998; Montain et al. 1998). Furthermore, it has recently been shown that dehydration amounting to 2.9 % of body weight increases muscle glycogen utilisation and muscle lactate accumulation during prolonged cycling exercise in a thermoneutral environment (Hargreaves et al. 1996b). However, the latter is in contrast to the unaltered rate of muscle glycogen depletion, muscle lactate accumulation and leg lactate release observed during prolonged exercise in the heat with smaller dehydration but higher hyperthermia (Nielsen et al. 1990, 1993). Moreover, recent evidence points to an unchanged muscle inorganic phosphate/β-ATP ratio and pH during exhaustive one-legged knee-extension exercise in markedly dehydrated subjects (Montain et al. 1998). Therefore, it remains unclear whether or not dehydration increases muscle lactate production and glycogen utilisation and whether these alterations are accompanied by a reciprocal reduction in muscle lipid metabolism. No previous study has directly determined the influence of dehydration on substrate delivery, fuel utilisation and energy turnover in active skeletal muscles during prolonged exercise. This is of particular interest because hyperthermia combined with dehydration result in a reduction in active skeletal muscle blood flow during prolonged exercise in the heat (González-Alonso et al. 1998).
We have previously reported that the marked reductions in systemic and exercising muscle blood flow with dehydration and hyperthermia are accompanied by unaltered O2 delivery and leg oxygen consumption rate (V̇O2) during the first 2 h of exercise (González-Alonso et al. 1998). Yet, at exhaustion in the dehydration trial, O2 delivery and leg V̇O2 were significantly reduced or tended to be lower compared with control. Substrate delivery to the exercising leg and the removal of locally produced muscle metabolites have been shown to be closely matched in conditions of adequate systemic and skeletal muscle blood flow (Ahlborg et al. 1974; Ahlborg & Felig, 1977; Nielsen et al. 1990, 1993; Kiens et al. 1993). However, the marked reductions in systemic and active skeletal muscle blood flow with dehydration could potentially reduce the delivery of substrate as well as the removal of locally produced muscle metabolites and heat. This condition could result in energy deficiency, metabolite accumulation and critically high muscle temperature, which in turn could limit intracellular metabolic processes and anticipate fatigue (Brooks et al. 1971; Sahlin et al. 1998).
Hyperthermia might be another plausible factor involved in the early fatigue with dehydration since exercise performance in the heat in trained cyclists has been shown to be closely associated with high internal body temperature (Nielsen et al. 1993). In a parallel study, we have recently observed that cyclists exercising at 60 % of maximal V̇O2 (V̇O2,max) fatigued at remarkably similar core and muscle temperatures (∼40 and ∼40.7°C, respectively), regardless of the wide differences in the initial value and the rate of rise of body temperature (González-Alonso et al. 1999). Since dehydration impairs heat dissipation and leads to a progressive increase in body temperature, it seems plausible that the attainment of a critically high body temperature contributes to the early fatigue observed with dehydration during prolonged exercise in the heat.
Therefore, the purpose of this study was to determine whether reductions in exercising skeletal muscle blood flow would alter substrate delivery to and metabolite and heat removal from the exercising leg and whether metabolic factors and/or hyperthermia are responsible for the early fatigue observed with dehydration during prolonged, moderately intense exercise in the heat.
METHODS
Subjects
Seven healthy endurance-trained males participated in this study. They had a mean (±s.d.) age of 27 ± 2 years, body weight of 78.1 ± 7.4 kg, height of 184 ± 7 cm, maximal heart rate of 197 ± 11 beats min−1 and V̇O2,max of 4.9 ± 0.6 l min−1 or 63 ± 5 ml kg−1 min−1. The subjects were fully informed of any risks and discomforts associated with the experiments before giving their informed written consent to participate. The study conformed to the code of Ethics of the World Medical Association (Declaration of Helsinki) and was approved by the Ethics Committee of Copenhagen and Frederiksberg communities. All subjects had participated in experiments involving endurance performance in hot environments before undergoing this experiment. Furthermore, subjects acclimated to the heat and adapted to large volumes of ingested fluid before the experimental trials by performing four practice trials (2 h cycling exercise at ∼60 % of V̇O2,max in a 35°C environment).
Experimental design
On two occasions separated by 1 week, subjects exercised continuously on a cycle ergometer (Electronic Monark 829E) in the heat (35°C; 40-50 % relative humidity, 1-2 m s−1 fan speed) at a power output (208 ± 21 W; 80-90 r.p.m.) that initially elicited 61 ± 2 % of V̇O2,max. In the first trial (dehydration trial, DE), they cycled until volitional exhaustion (135 ± 4 min (±s.e.m.); range 122-148 min), while developing significant dehydration and hyperthermia (3.9 ± 0.3 % body weight loss, 39.7 ± 0.2°C oesophageal temperature, Toes). In the second trial (control), they cycled for the same period of time while maintaining euhydration by consuming fluids and stabilising Toes at 38.2°C after 30 min of exercise. To ensure this large difference in core temperature, fan speed was doubled in the control trial. During DE, subjects only received ∼0.8 l of fluid: (1) 0.2 l of a concentrated carbohydrate-electrolyte solution by mouth and (2) ∼0.6 l of a saline solution (0.9 % NaCl) intravenously infused during the leg blood flow measurements. In so doing, they replaced 28 % of the sweat losses and lost 3.07 ± 0.28 kg or 3.9 ± 0.3 % of their body weight. During control, subjects received ∼4.3 l of fluid: (1) 3.7 ± 0.1 l of a diluted carbohydrate-electrolyte solution by mouth and (2) ∼0.6 l of intravenously infused saline. This resulted in the maintenance of body weight throughout the experimental trial. The same amount of carbohydrate and electrolytes was ingested in each trial (i.e. 99 g of glucose and 1 g of a mixture containing Na+, Cl− and K+). During control, the 3.7 l of fluid was divided into equal aliquots and ingested at 20, 35, 50, 65, 80, 95 and 110 min of exercise. During DE, the 0.2 l of fluid was ingested at 30, 60 and 90 min of exercise. The estimated average sweating rate was 1.5 ± 0.1 l h−1 in both trials. The temperature of the oral fluid replacement was ∼38°C to minimise fluctuations in core temperature.
On the day prior to experimental testing, the hydration status of the subjects was standardised by having them adopt the same diet, exercise bout (1 h of low intensity cycling) and fluid intake regimen. The subjects reported to the laboratory approximately 3-4 h prior to the experiment, following the ingestion of a plentiful breakfast (consisting of several pieces of bread, marmalade, cookies, cereals, tea, milk) and 200-300 ml of fluid (water or juice). Upon arrival, they rested in the supine position while two catheters were placed, using the Seldinger technique, one in the femoral vein and another in the femoral artery of the right leg. Both catheters were positioned 1-2 cm distal from the inguinal ligament (antegrade position). A thermistor (model 94-030-2.5F T. D. Probe, Edwards Edslab, Baxter, Irvine, CA, USA) to measure venous blood temperature was inserted through a catheter. Furthermore, a thermocouple (model MAC-07170-A, Ellab, Denmark) to measure muscle temperature was inserted through a Venflon (Ohmeda AB, Helsingborg, Sweden) catheter 3-4 cm into the vastus lateralis. A second thermistor was inserted in an antecubital vein to measure the venous blood temperature of a resting limb. A resting muscle biopsy from the vastus lateralis was then obtained.
Thereafter, the subjects walked to another room, were weighed and sat in an armchair while electrodes to measure 3-lead electrocardiogram and skin thermistors (model MHC-40050-A, Ellab) to measure temperature at six sites (upper arm, forearm, chest, back, thigh and calf) were attached. A thermocouple (model MOV-05005-A, Ellab) to measure core (oesophageal) temperature was then inserted through the nasal passage a distance equal to one-fourth of the subject's standing height. After 15 min of seated rest, 10 ml blood samples were withdrawn simultaneously from the femoral artery and vein for later determination of baseline blood variables. The subjects then entered an environmental chamber (35°C and 40-50 % relative humidity) and sat on a cycle ergometer while resting cardiovascular and temperature measurements were obtained. They then started to exercise.
During exercise, heart rate, arterial blood pressure and skin, core, blood and muscle temperature were recorded continuously. Pulmonary V̇O2 was measured during a 5 min period beginning at 5, 40, 80, 105 and 125-135 min of exercise. Leg blood flow (themodilution) was measured in duplicate at 20, 50, 90, 120 and 135 ± 4 min of exercise. Arterial and venous blood samples (10 ml) were withdrawn simultaneously at 20, 60, 90, 120 and 135 ± 4 min of exercise. A rating of perceived exertion was recorded after 30, 60, 90, 120 and 135 ± 4 min of exercise using the Borg scale (Borg, 1975). Upon completion of the exercise, a post-exercise muscle biopsy was obtained within 2-4 min. Thereafter, catheters and thermocouples were removed and post-exercise naked body weight was recorded.
An additional dehydration experiment was performed in one subject while measuring femoral arterial and venous blood temperature, oesophageal temperature and leg blood flow during exercise until fatigue (208 W and 85 r.p.m.). The aim was to determine the difference between oesophageal temperature and femoral arterial blood temperatures during steady-state exercise to calculate convective heat transfer from the exercising leg to the body core in the main study.
Leg blood flow
Femoral venous blood flow (i.e. leg blood flow, LBF) was determined by the constant infusion thermodilution technique, originally described by Andersen & Saltin (1985) and was modified in this study (González-Alonso et al. 1998).
Core, skin, muscle temperature and blood temperature
The oesophageal, muscle and skin thermocouples were connected to a recorder (CTF 90008 precision thermometer, Ellab) interfaced to an IBM-AT computer. Blood temperatures were recorded using a data acquisition system (MacLab 8:s, ADInstruments, Sydney, Australia) interfaced to a computer (Macintosh Performa). After each experiment, all thermistors and thermocouples were calibrated with a mercury thermometer with a precision of 0.05°C.
Blood analysis
Blood glucose and blood lactate were analysed on a glucose and lactate analyser (Yellow Springs Instruments, Yellow Springs, OH, USA). Plasma glycerol was determined on an automatic analyser (Cobas Fara, Roche, France). Plasma free fatty acid (FFA) was measured fluorometrically using an enzymatic kit (WAKO Chemical, Germany). Haemotocrit (Hct) was measured in triplicate after micro-centrifugation and corrected for trapped plasma (0.98). Haemoglobin concentration and blood O2 saturation were determined spectrophotometrically (Radiometer, OSM-2 Hemoximeter, Copenhagen, Denmark). PO2, PCO2 and pH were determined with the Astrup technique (ABL30, Radiometer) and corrected for measured blood temperature. Plasma noradrenaline and adrenaline concentrations were determined using a radio enzymatic assay (Christiansen et al. 1980). Plasma insulin and plasma glucagon were determined using commercially available radioimmunoassay kits (Pharmacia Insulin RIA 100, Sweden and Linco's Glucagon RIA, Linco Research Inc., St Louis, MO, USA).
Muscle samples analysis
The biopsy samples were frozen in liquid nitrogen within 5-10 s and stored at -80°C until analysis. Muscle biopsies were first analysed for total water content by weighing the samples before and after freeze drying. They were subsequently homogenised and analysed for lactate, creatine phosphate (PCr) and glycogen using fluorometric assays (Lowry & Passonneau, 1972).
Calculations
Estimation of blood CO2 content
Arterial and venous whole-blood CO2 content was determined from blood haemoglobin, temperature, O2 saturation, pH and PCO2 according to the model proposed by Douglas et al. (1988).
Substrate delivery to the exercising leg
Substrate delivery was calculated by multiplying blood flow (or plasma flow for FFA) by the arterial concentration of a given substrate.
Leg V̇O2, V̇CO2 and substrate exchange
V̇O2 and CO2 production (V̇CO2), and net lactate, net glucose, net FFA and net glycerol exchange by the leg were calculated by multiplying blood flow or plasma flow by the difference between the femoral artery and vein concentrations of O2, glucose, FFA and glycerol (e.g. a-v O2 difference) or the difference between the femoral vein and artery concentrations of CO2 and lactate (e.g. v-a CO2 difference). To correct for changes in plasma volume, venous substrate values were multiplied by the ratio of arterial-to-venous haemotocrit. In a preliminary experiment in one subject during submaximal cycle ergometer exercise, we observed very similar concentrations of lactate, glucose, FFA and glycerol in the femoral vein and the great saphenous vein, which drains the leg skin and subcutaneous fat, when leg skin blood flow was either low or high (0.08 vs. 0.47 l min−1, respectively) and LBF was 7-8 l min−1. It is therefore assumed that the measured leg substrate exchange during cycling exercise reflects metabolic changes in the muscle compartment. The leg respiratory quotient (RQ) was calculated as the ratio between leg V̇CO2 and leg V̇O2.
Total V̇O2, total V̇CO2 and total substrate exchange
The total V̇O2, total V̇CO2, total lactate, total glucose and total FFA exchange during the 20 to 135 ± 4 min period of exercise in DE and control trials were calculated by the time integral:
where x is 20 min, y is 122-148 min, and f(t) is the exchange of any of these variables at a given time (t) during exercise. Total exchanges were calculated from the areas under the f(t) curves, with time on the X-axis. The curves were constructed assuming a linear relationship with time in the interval between two consecutive measured values.
Total leg energy turnover
Total leg carbohydrate and fat oxidation was calculated from non-protein leg RQ and leg V̇O2 (Péronnet & Massicotte, 1991). The few RQ values that were slightly higher (1-5 %) than 1.00 were considered to reflect full contribution of carbohydrate to total energy production. The estimated contribution of protein during prolonged exercise amounted to approximately 2 % of the total fuel utilisation and therefore was disregarded (Graham et al. 1991). The uptakes of plasma glucose and FFA by the leg during the 20 to 135 ± 4 min period of exercise were converted into their energy equivalents and used as an index of maximal contribution of these plasma substrates to energy. The minimal intramuscular and circulating triacylglycerol oxidation was calculated as the difference between total leg fat oxidation and FFA oxidation. The difference between total leg carbohydrate oxidation and plasma glucose uptake was used as the index of minimal glycogen oxidation (Romijn et al. 1993). Total leg energy turnover was estimated as the sum of aerobic and anaerobic energy turnover. The small anaerobic energy turnover during exercise was estimated by adding the net muscle lactate accumulation, the net lactate release and net muscle PCr degradation.
Heat transfer from the leg to the body core
The rate of heat transfer from the active leg muscles to the body core was calculated by multiplying the mean venous-arterial (v-a) temperature difference by LBF and the specific heat of the blood at 38.5°C (3.6 kJ l−1°C−1; Hct ∼45 %). Femoral arterial blood temperature was estimated from oesophageal temperature values, using a correction factor (-0.17°C) obtained in the additional experiment. The mean leg heat production was calculated by subtracting the heat equivalent of power output from the heat equivalent of total metabolic energy turnover.
Statistical analysis
A two-way (trial-by-time) repeated measures analysis of variance (ANOVA) was performed to test for significant differences between and within treatments. Following a significant F test, pair-wise differences were identified using Tukey's honestly significant difference (HSD) post hoc procedure. When appropriate, significant differences were also identified using Student's paired t tests. The significance level was set at P < 0.05. Data are presented as means ±s.e.m. unless otherwise stated.
RESULTS
Hydration status, leg blood flow, baseline metabolism and body temperature
Hydration was equal in both trials as indicated by similar body weights (mean range between trials, 78.5-78.8 (± 2.8) kg) and arterial and femoral venous haemoglobin concentration (mean range between trials, 8.8-8.9 (± 0.3) mmol l−1). In addition, other indicators of hydration status such as haemotocrit, osmolality and total plasma proteins were also similar between trials at rest and after 20 min of exercise. Between-trial differences in hydration were only present after 30-50 min of exercise and generally became significant after the first hour of exercise, reaching a difference of 4 % body weight by the end of exercise. During DE, LBF declined significantly during the 20 to 135 ± 4 min period of exercise (7.5 ± 0.4 to 6.8 ± 0.4 l min−1; P < 0.05). In contrast, LBF tended to increase during the same time period of control (7.4 ± 0.4 to 7.8 ± 0.4 l min−1; n.s.). Therefore, after 120 and 135 ± 4 min of DE, LBF was 0.6 ± 0.2 and 1.0 ± 0.3 l min−1 lower, respectively, compared with control (P < 0.05) (for further results see González-Alonso et al. 1998). Leg substrate exchange and circulating levels of glucose, lactate, FFA, glycerol, catecholamines, insulin and glucagon were also similar in both trials at rest and during the first 20 min of exercise (Fig. 1). Furthermore, whole-body V̇O2, leg V̇O2 and temperatures of muscle, skin, blood and core were also similar.
Figure 1. Arterial substrate concentrations during the dehydration and control trials.
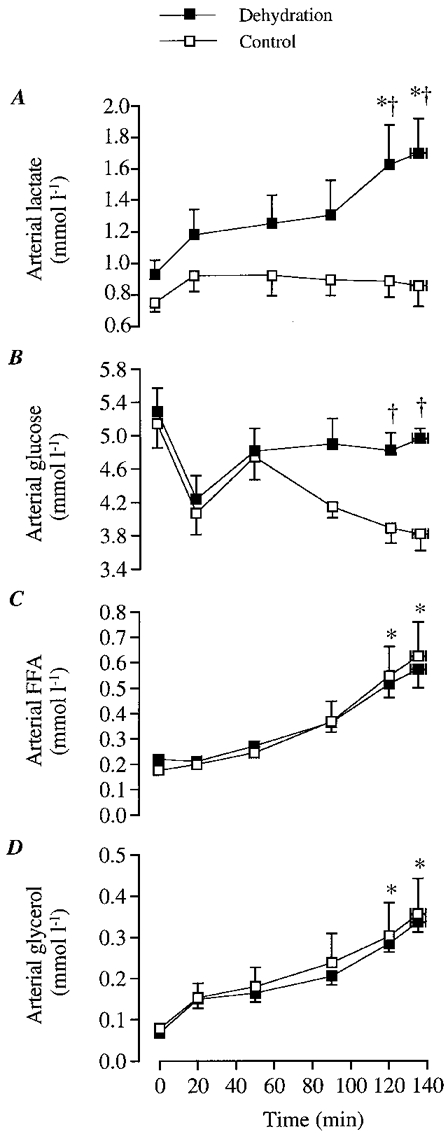
A, arterial lactate concentration; B, arterial glucose concentration; C, arterial FFA concentration; D, arterial glycerol concentration. * Significantly higher than 20 min value, P < 0.05. † Significantly higher than control, P < 0.05.
Arterial concentrations of lactate, glucose, FFA and glycerol
During DE, arterial lactate concentration increased over time and was higher (P < 0.05) after 120 min (1.7 ± 0.2 mmol l−1) compared with 20 min of exercise (1.2 ± 0.2 mmol l−1). In contrast, arterial lactate concentration did not increase throughout the control trial (Fig. 1A). Thus, after 2 h of exercise, arterial lactate concentration was higher during DE (1.7 ± 0.2 mmol l−1) compared with control (0.9 ± 0.1 mmol l−1; P < 0.05; Fig. 1A).
In both trials, the arterial glucose concentration was similar during the first hour of exercise (Fig. 1B). Thereafter, arterial glucose was maintained at approximately 5 mmol l−1 during DE but declined progressively to 3.8 ± 0.2 mmol l−1 during control. Thus, arterial glucose concentration was higher after 2 h of exercise in DE compared with control (Fig. 1B). Consequently, leg glucose delivery was also higher during DE compared with control at the end of exercise despite reduced LBF (34-35 (± 3) vs. 30 ± 3 mmol min−1, respectively; P < 0.05).
Arterial FFA concentration increased from 0.2 mmol l−1 at 20 min of exercise to above 0.6 mmol l−1 after 2 h of exercise in both trials (P < 0.05), and thus leg FFA delivery increased similarly from 0.9 to 2.5-2.8 mmol min−1 (P < 0.05; Fig. 1C). During the same time period, arterial glycerol concentration increased from 0.15 mmol l−1 to 0.34 mmol l−1 in both trials (P < 0.05; Fig. 1D).
Substrate exchange across the leg
During the first 90 min of exercise, leg net lactate release was 0.3-0.4 mmol min−1 in both trials (Fig. 2A). However, net lactate release increased abruptly to 0.9 mmol min−1 (P < 0.05) during the last 15 min of DE, while it remained unaltered during the same time in control. Therefore, net lactate release at the end of exercise was higher during DE (0.9 ± 0.2 mmol min−1) compared with control (0.3 ± 0.1 mmol min−1; Fig. 2A). The higher net lactate release during DE was due to an increase in femoral v-a lactate difference (0.06 ± 0.2 vs. 0.13 ± 0.02 mmol l−1 at 20 vs. 135 min, respectively; P < 0.05), which was much greater than the concomitant reduction in LBF. In contrast, femoral v-a lactate difference during control was maintained at 0.04-0.06 (± 0.2) mmol l−1 throughout exercise. During the 20 to 135 ± 4 min period of exercise, total lactate release was higher during DE compared with control (91 ± 22 vs. 60 ± 10 mmol, respectively; P < 0.05).
Figure 2. Leg substrate exchange during the dehydration and control trials.
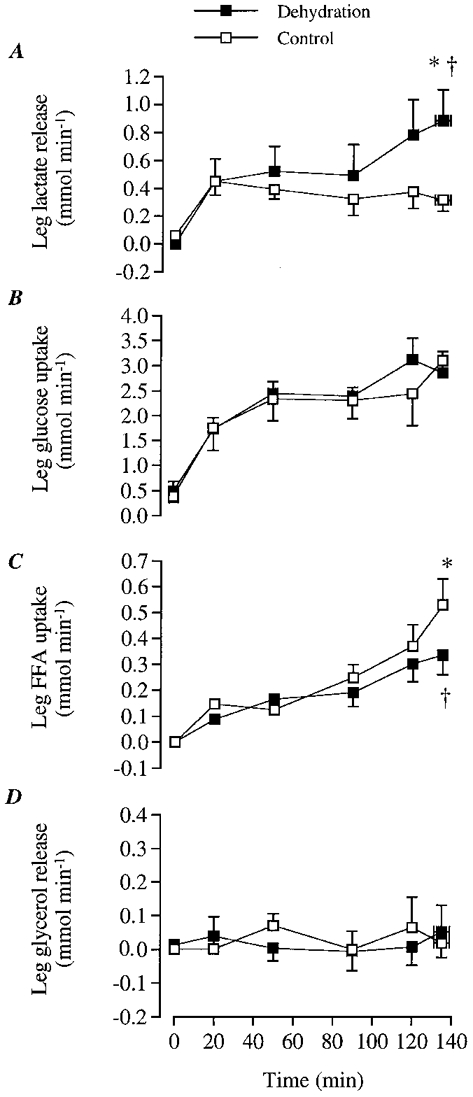
A, leg lactate release; B, leg glucose uptake; C, leg FFA uptake; D, leg glycerol release. * Significantly higher than 20 min value, P < 0.05. † Significantly lower than control, P < 0.05.
Despite the higher leg glucose delivery during DE compared with control after the first hour of exercise (∼4-5 mmol min−1 or 13-17 % higher; P < 0.05), leg glucose uptake was not different between trials at any time point (Fig. 2B). In both trials, leg glucose uptake increased over time (1.8 ± 0.4 vs. 3.1 ± 0.4 mmol min−1 after 20 vs. 120 min, respectively; P < 0.05; Fig. 2B). This was due to the increase over time in femoral a-v glucose difference (0.23 vs. 0.39-0.43 mmol l−1 at 20 vs. 135 ± 4 min, respectively; P < 0.05). During the 20 to 135 ± 4 min period of exercise, total glucose uptake was similar during DE to control (392 ± 29 vs. 390 ± 54 mmol, respectively).
Leg net FFA uptake increased over time during control (0.15 ± 0.05 vs. 0.53 ± 0.10 mmol l−1 at 20 vs. 135 (± 4) min, respectively; P < 0.05). During DE, there was also a trend for an elevation in net FFA uptake (0.09 ± 0.01 vs. 0.33 ± 0.08 mmol l−1 at 20 vs. 135 (± 4) min, respectively; n.s.; Fig. 2C). However, at the end of exercise, net FFA uptake was significantly lower during DE compared with control (Fig. 2C). This was largely due to the smaller increase over time (20 to 135 ± 4 min) in femoral a-v FFA difference during DE compared with control (0.06 ± 0.01 vs. 0.09 ± 0.01 mmol l−1, respectively; P < 0.05). During the 20 to 135 ± 4 min period of exercise, total FFA uptake was significantly lower during DE compared with control (33 ± 8 vs. 40 ± 7 mmol, respectively; P < 0.05). In contrast, leg glycerol release was not significantly different either over time or between DE and control (Fig. 2D).
Leg RQ, leg CO2 release and leg O2 consumption
Estimated leg RQ was significantly higher during DE compared with control at 2 h of exercise (Fig. 3A). The mean leg RQ during the 20 to 135 ± 4 min period of exercise tended to be higher during DE compared with control (0.94 ± 0.01 vs. 0.92 ± 0.01; P= 0.07). At the systemic level, the mean respiratory exchange ratio (RER) was significantly higher during DE (0.93 ± 0.01) compared with control (0.90 ± 0.01).
Figure 3. Leg respiratory quotient, carbon dioxide production and oxygen consumption during the dehydration and control trials.
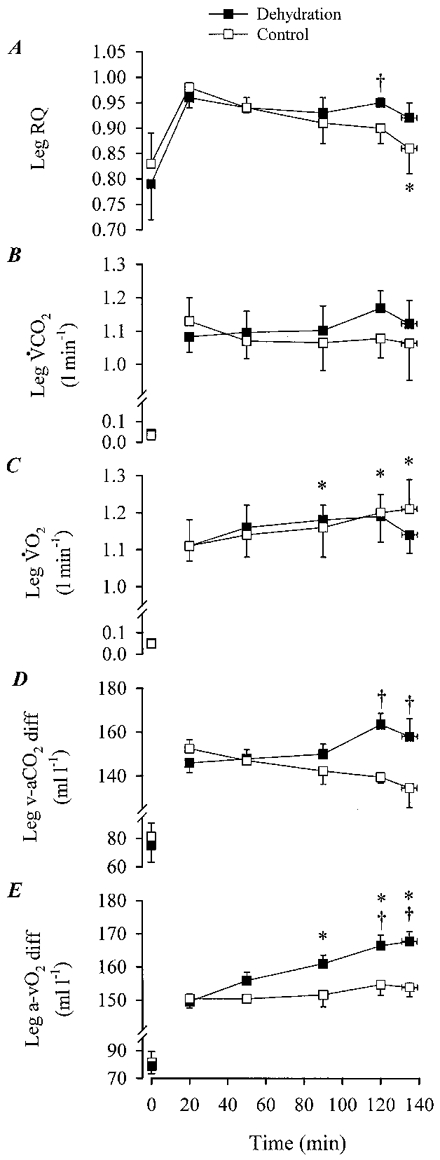
A, leg RQ; B, leg VCO2; C, leg VO2; D, femoral venous-arterial CO2 (v-aCO2) difference; E, femoral arterial-venous O2 (a-vO2) difference during the dehydration and control trials. * Significantly different from the 20 min value during the dehydration trial, P < 0.05. † Significantly different from control, P < 0.05.
During the 20-120 min period of exercise with DE, femoral v-a CO2 difference tended to increase progressively from 146 ± 40 to 163 ± 50 ml l−1, respectively (Fig. 3D). In contrast, during control, femoral v-a CO2 difference tended to decline after 120 min (135 ± 90 ml l−1) compared with the 20 min of exercise (152 ± 40 ml l−1; Fig. 3D). Therefore, femoral v-a CO2 difference during DE was significantly (P < 0.05) higher than control after 2 h of exercise. Accordingly, leg V̇CO2 release tended to be higher in DE compared with control after the 2 h of exercise (Fig. 3B). During the 20 to 135 ± 4 min period of exercise, total leg V̇CO2 tended to be higher during DE compared with control (181 ± 10 vs. 178 ± 13 l, respectively; n.s.).
During the 20 to 135 ± 4 min period of exercise with DE, femoral a-v O2 difference increased progressively from 150 ± 50 to 168 ± 80 ml l−1, respectively (P < 0.05; Fig. 3E). During control, femoral a-v O2 difference was also significantly higher after 120 min of exercise compared with the 20 min value (150 ± 40 vs. 155 ± 90 ml l−1, respectively; P < 0.05; Fig. 3E). Nevertheless, after 50 min of exercise, femoral a-v O2 difference during DE was significantly (P < 0.05) higher than control. In both trials, leg V̇O2 increased significantly (P < 0.05) during the 20-120 min period of exercise (i.e. 0.08-0.10 l min−1; Fig. 3C). At exhaustion in DE, there was a trend for leg V̇O2 to decline compared with control (5 ± 2 %, n.s.; Fig. 3C). Nevertheless, during the 20 to 135 ± 4 min period of exercise, total leg V̇O2 was the same in both trials (i.e. 192 ± 11 l).
Muscle glycogen, lactate and creatine phosphate
Before exercise, muscle glycogen, lactate and PCr contents were similar in both trials. After exercise, however, muscle glycogen content was significantly lower and muscle lactate content was higher in DE compared with control (Fig. 4A and B). Therefore, net glycogen utilisation during exercise was higher during DE compared with control (298 ± 21 vs. 205 ± 15 mmol (kg dry weight)−1, respectively; P < 0.05). Furthermore, net muscle lactate accumulation during exercise was higher during DE compared with control (5.0 ± 1.9 vs. 0.9 ± 0.7 mmol (kg dry weight)−1, respectively; P < 0.05). PCr degradation during exercise was low in both trials based on the observation that the 2-4 min post-exercise PCr values were similar to the initial 82-83 (± 3) mmol (kg dry weight)−1 resting values.
Figure 4. Glycogen and lactate content of vastus lateralis muscle during dehydration and control trials.
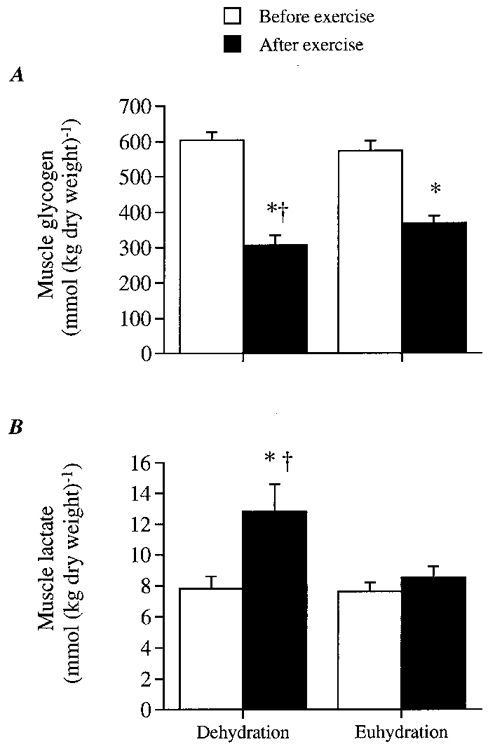
A, muscle glycogen content; B, lactate content. * Significantly different from 20 min value, P < 0.05. † Significantly different from control, P < 0.05.
Leg lipid and carbohydrate oxidation estimated from leg V̇O2 and leg RQ
During DE, leg carbohydrate oxidation was maintained at 1.1-1.2 g min−1. In contrast, leg carbohydrate oxidation tended to decline from 1.3 ± 0.1 to 0.9 ± 0.2 g min−1 during control (P= 0.08). Therefore, leg carbohydrate oxidation tended to be higher after 90 min of DE compared with control. Total leg carbohydrate oxidation during the 20-135 min period of exercise was higher during DE vs. control (183 ± 16 vs. 169 ± 18 g; P < 0.05). Reciprocally, leg lipid oxidation during the 20-135 min period of exercise was maintained during DE whereas it tended to increase during control (0.04 ± 0.02 to 0.24 ± 0.08 g, respectively; P= 0.09). Total leg lipid oxidation was significantly lower during DE (21 ± 4 g) compared with control (27 ± 5 g).
Leg energy turnover estimated from leg V̇O2, leg RQ, lactate and PCr
During the first 2 h of exercise, leg energy turnover increased significantly over time in both trials (6-7 (± 2) %; P < 0.05). At exhaustion, leg energy turnover tended to be lower during DE compared with control (5 %; n.s.; Fig. 5A and B). However, during the 20 to 135 ± 4 min period of exercise, no significant difference in total leg energy turnover was observed between DE and control (3990 ± 221 vs. 3968 ± 307 kJ, respectively, n.s.). Nevertheless, anaerobic energy turnover estimated from net muscle lactate accumulation (assuming 5 kg of active muscle mass in the leg) and net leg lactate release were significantly higher during DE compared with control (3.4 ± 0.6 vs. 1.6 ± 0.3 kJ, respectively; P < 0.05). It is estimated that PCr degradation averaged ∼0.03 kJ min−1 throughout exercise (Hargreaves et al. 1996b). Collectively, the between-trial difference in anaerobic energy turnover and its overall contribution to total leg energy turnover (i.e. < 0.3 %) is clearly negligible in terms of total ATP energy turnover. During the 20 to 135 ± 4 min period of exercise, total carbohydrate oxidation was 8 % higher during DE compared with control (3162 ± 251 vs. 2937 ± 301 kJ, respectively; P < 0.05). Therefore, carbohydrate oxidation contributed to 74-79 % of the total leg energy turnover during the 20 to 135 ± 4 min period of exercise in both trials.
Figure 5. Estimated maximal contribution of blood-borne glucose and FFA, and minimal contribution of intramuscular glycogen and circulating and muscle triacylglycerol (TG) to total leg energy production.
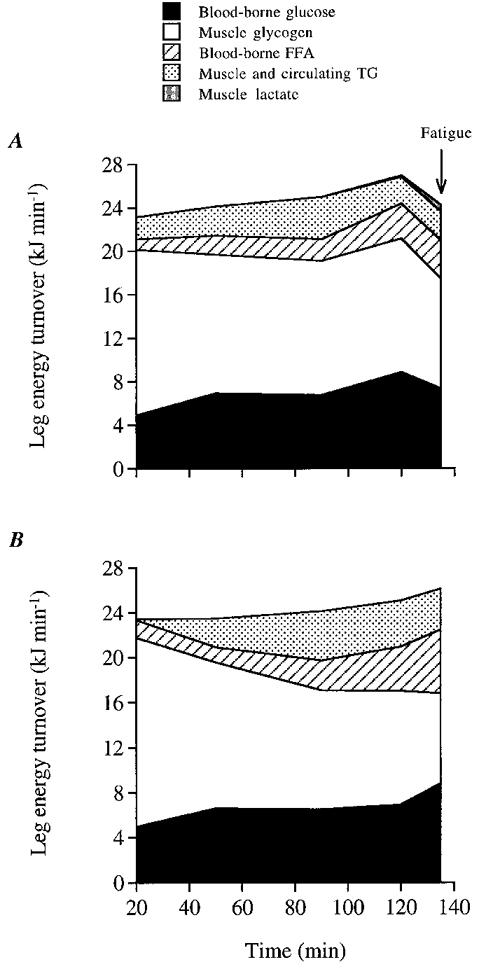
Levels throughout the dehydration (A) and control (B) trials. Lactate contribution was estimated by adding net leg lactate release and net muscle lactate accumulation. The latter was estimated assuming an active muscle mass in the leg of 5 kg and correcting for the lactate release (≈1.5 mmol) occurring during the 2-4 min before the biopsy was obtained (Bangsbo et al. 1994).
Arterial concentrations of insulin, glucagon and catecholamines
Arterial insulin concentration was only higher in control compared with DE at 50 min of exercise (10.8 vs. 7.7 μU ml−1, respectively; P < 0.05). However, during exercise in both trials, arterial insulin concentration tended to decline from 8.0-8.2 μU ml−1 at 20 min of exercise to 6.8-7.0 μU ml−1 after 135 ± 4 min (n.s.; Fig. 6A).
Figure 6. Plasma insulin, plasma glucagon, arterial noradrenaline and arterial adrenaline during the dehydration and control trials.
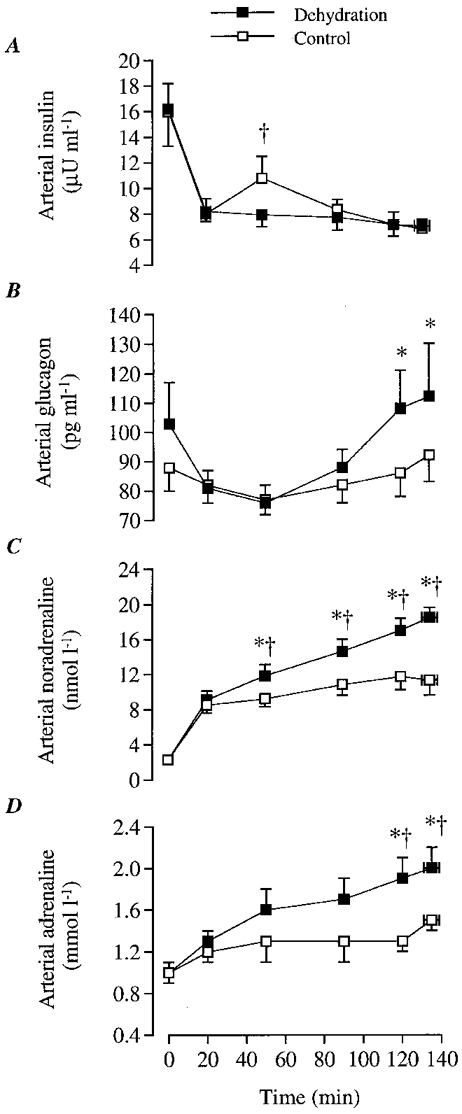
A, plasma insulin concentration; B, plasma glucagon concentration; C, arterial noradrenaline concentration; D, arterial adrenaline concentration; E, vastus lateralis muscle temperature. * Significantly different from 20 min value, P < 0.05. † Significantly different from control, P < 0.05.
During DE arterial glucagon concentration increased after 2 h (108-112 (± 15) pg ml−1) compared with 20 min of exercise (81 ± 6 pg ml−1; P < 0.05). In contrast, arterial glucagon concentration did not increase during control. Thus, arterial glucagon tended to be higher during DE compared with control after 90 min of exercise (Fig. 6B).
During DE, arterial noradrenaline and adrenaline concentrations increased progressively and were significantly elevated above the 20 min value after the first hour of exercise. In contrast, during control arterial adrenaline did not increase significantly whereas an elevation in noradrenaline was observed at the end compared with 20 min of exercise. Thus, noradrenaline and adrenaline were higher during DE compared with control after the first hour of exercise (Fig. 6C and D).
Body temperatures and convective heat transfer from the leg
During DE, oesophageal, femoral venous blood and muscle temperatures increased progressively throughout exercise up to 39.7-39.8 (± 0.2)°C for oesophageal and femoral venous blood temperature and 40.4 (± 0.2)°C for muscle temperature (Fig. 7A). In contrast, oesophageal and femoral venous blood temperature were maintained at 38.1-38.3°C and muscle temperature at 38.5-38.8°C throughout the control trial. Therefore, oesophageal, femoral venous blood and muscle temperatures were significantly higher during DE compared with control after 40 min of exercise, all being 1.5°C higher in DE compared with control at the end of exercise (Fig. 7A and B). Conversely, venous blood temperature in the resting forearm was 35.3-35.5 (± 0.3)°C at 20 min of exercise in both trials increasing 0.9-1.2°C thereafter. Similarly, mean skin temperature was 34.1-34.6 (± 0.3)°C at 20 min of exercise increasing 0.6-0.8°C in both trials. Mean skin temperature was ∼0.5°C higher throughout exercise in DE compared with control (P < 0.05).
Figure 7. Body temperature during dehydration and control trials.
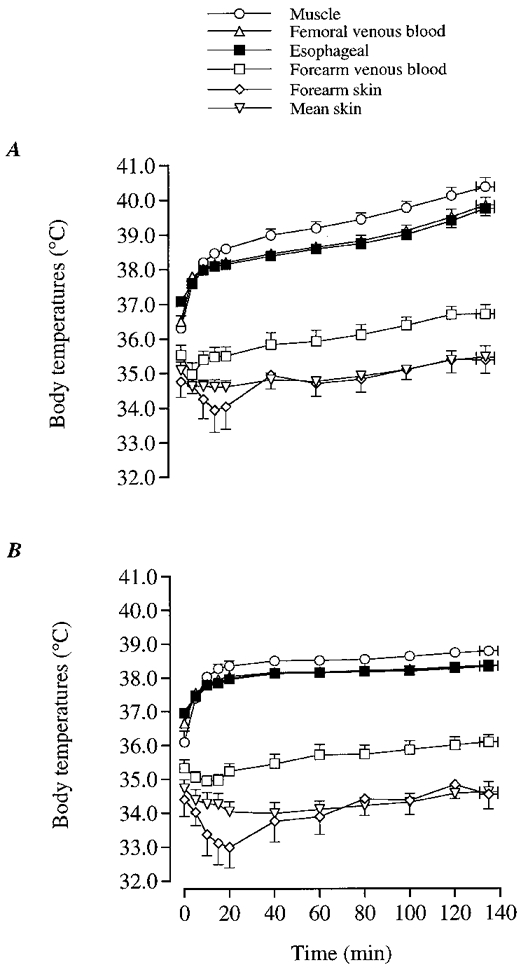
Temperatures of muscle, oesophagus, femoral venous blood, forearm venous blood, forearm skin and whole-body skin during dehydration (A) and control (B) trials.
There were no differences over time in convective heat transfer from the leg in either trial. The estimated mean femoral v-a temperature difference throughout exercise was 0.21 ± 0.06°C in control and 0.26 ± 0.05°C in DE whereas the mean convective heat transfer from the leg to the body core was 6.3 ± 1.7 kJ min−1 in control and 7.2 ± 1.3 kJ min−1 in DE. In the additional experiment, the mean femoral v-a temperature difference was 0.38°C, whereas the average convective heat transfer to the core was 7.6 kJ min−1.
Rating of perceived exertion
At 30 min of exercise, the rating of perceived exertion (RPE) was similar in both trials (11-12 units; i.e. fairly light). During DE, RPE increased progressively to 19.4 ± 0.2 units (i.e. very, very hard) at exhaustion. In contrast, during control, RPE only increased to 13.6 ± 0.6 units (i.e. somewhat hard).
DISCUSSION
This study tested the hypothesis that reduced blood flow to contracting skeletal muscles alters substrate delivery, fuel selection and metabolite removal and that these alterations, together with hyperthermia, contribute to the early fatigue observed with dehydration during prolonged exercise in the heat. We found that lower leg muscle blood flow with dehydration and hyperthermia did not alter either the delivery of glucose and FFA or impair the removal of lactate. However, dehydration reduced net FFA uptake and increased muscle glycogen utilisation and lactate production compared with control. Despite altered metabolism, fatigue with dehydration appears largely related to hyperthermia.
Effect of reduced leg blood flow on substrate delivery, net substrate exchange and lactate removal
An important finding of this study was that reductions in LBF of up to 1 l min−1 (13 %) with dehydration and hyperthermia did not compromise either the delivery to the contracting leg muscles of glucose and FFA or the removal of lactate compared with control. This was due to the fact that the lower LBF after 2 h of exercise was accompanied by proportional or greater elevations in arterial concentrations of glucose and FFA and venous concentrations of lactate. This observation indicates that glucose and FFA availability did not limit the uptake and oxidation of these substrates in the exercising leg muscles when the subjects were dehydrated.
Interestingly, leg glucose uptake increased similarly throughout exercise in both trials, despite the 13-17 % higher leg glucose delivery at the end of exercise in DE compared with control (see Fig. 1B). This finding is consistent with the observation that arterial insulin concentrations were the same during most of the exercise period, suggesting a similar stimulus for insulin-stimulated glucose uptake in the leg muscles with dehydration (Fig. 6A). The higher leg glucose delivery in DE was largely due to the maintainance of arterial glucose concentration at ∼5 mmol l−1 with dehydration but declined to 3.8 mmol l−1 in the control trial. The gradual lowering in arterial glucose concentration after 50 min exercise during control, when leg glucose uptake increased ∼70 % throughout exercise in both trials, appears to be the result of a blunting or lack of a corresponding increase in hepatic glucose production. An exaggerated increase in glucose disposal by other upper body tissues appears to be a less likely possibility given the constancy of non-legged V̇O2 and the significant progressive decline in RER. A suppressed liver glucose output in control could in part be ascribed to a lack of increase in sympathetic activity and glucagon action, as suggested by the unaltered circulating catecholamines and blunted rise in glucagon compared with dehydration (see Fig. 6) (Kjær et al. 1991, 1993; Hargreaves et al. 1996a).
On the other hand, dehydration blunted the progressive increase in leg FFA uptake after 90 min of exercise compared with control, thereby resulting in a 17 % lower net FFA uptake (Fig. 2C). The mean fractional FFA uptake was also lower in DE compared with control (12 ± 2 vs. 16 ± 2 %, respectively; P < 0.05). Because FFA delivery was unaltered by dehydration, the reduced leg net FFA uptake appears to result from an inhibition in FFA transport and/or intracellular uptake, possibly in response to a shift in fuel selection (Coyle et al. 1997). In support of this the lower net muscle FFA uptake coincided with an abrupt increase in leg lactate release, femoral v-a CO2 difference and leg RQ as well as elevated glycogen utilisation.
Effect of dehydration on substrate oxidation
Dehydration was shown to potentiate muscle glycogen utilisation, with neither apparent alteration in blood glucose contribution nor noticeable differences in total leg energy turnover during the first 2 h of exercise compared with control (see Fig. 5A and B). The progressive lowering in muscle glycogen breakdown during exercise was offset by larger increases in the oxidation of blood-borne substrates (glucose and FFA), so that total leg energy turnover increased 6-7 % over time in both trials. The estimated total leg carbohydrate oxidation during the 20-135 min period of exercise was 8 % higher with dehydration (183 ± 16 g) compared with control (169 ± 18 g). Assuming that all glucose taken up by active leg muscles is oxidised, it is estimated that the maximal contribution of blood glucose to total leg energy turnover was 28 % in both trials whereas the minimal contribution of muscle glycogen ranged from 46 to 51 % in the control and dehydration trials, respectively (Fig. 5A and B). These values of blood glucose and muscle glycogen utilisation during moderate intensity prolonged exercise are consistent with those reported in fed subjects (Bergman et al. 1999) but predictably are much greater than those estimated in subjects fasted overnight (Montain et al. 1991; Romijn et al. 1993).
The oxidation of blood-borne FFA appears to contribute to approximately one-half of leg lipid oxidation, which accounted for 21-26 % of total leg energy turnover in both the dehydration and control trials (Fig. 5A and B). The finding that leg glycerol release was within 0.00-0.07 mmol min−1 at rest and throughout exercise in both trials indeed supports the idea that lipolysis in fat depots located inside and outside the muscle fibres and in subcutaneous adipose tissue accounted for a small percentage of total leg energy turnover. Our preliminary results showing similar glycerol concentrations in the saphenous vein, which drains the leg skin and subcutaneous adipose tissue, and in the femoral vein, when cycling in the heat at similar work intensity, supports this argument. Furthermore, this is in agreement with the present estimation of a 10-12 % minimal contribution of both triacylglycerols transported via lipoproteins and intramuscular triacylglycerol stores in both trials (Fig. 5A and B). Given that the circulating very low density lipoprotein triacylglycerols might have contributed to at least part of this already low relative total energy contribution (Kiens et al. 1993), it is reasonable to conclude that the contribution of muscle triacylglycerol breakdown to total leg energy turnover was small in both the euhydrated and dehydrated state.
Factors mediating the increased muscle glycogenolysis with dehydration
There are at least three potential factors that could have stimulated the greater leg muscle glycogenolysis during dehydration compared with control: (i) reduced O2 supply and lower cytoplasmic PO2 (Wilson et al. 1977), (ii) increased muscle temperature (Q10) (Febbraio et al. 1996), and (iii) elevated circulating adrenaline (Febbraio et al. 1998). These factors have been suggested to increase muscle glycogenolysis by stimulating the activity of phosphorylase and other key enzymes involved in glycogen breakdown (Wilson et al. 1977; Febbraio et al. 1996, 1998). The finding that leg O2 delivery and leg V̇O2 were maintained at control levels during the first 2 h of exercise in DE would suggest that low intracellular PO2 did not play a major role in triggering the further activation of muscle glycogenolysis with dehydration. However, the possibility still exists that a lower cytoplasmic PO2, as suggested by slightly reduced femoral venous PO2 after 90 min of cycling (10.9 vs. 12.2 mmHg), might have contributed to some extent to the greater muscle lactate production and glycogen utilisation with dehydration during the last 45 min of exercise (Wilson et al. 1977). Indeed, a significantly lower intracellular PO2, paralleling a reduced femoral venous PO2 and increased arterial lactate concentration, has been reported with hypoxia compared with normoxia (2 vs. 3 mmHg) during dynamic knee-extension exercise (Richardson et al. 1995). Inasmuch as the present intravascular PO2 differences were small, the elevated muscle temperature and circulating adrenaline already existing after 50 min of exercise might instead largely explain the higher muscle glycogenolysis and possibly higher glycolysis with dehydration (Figs 6B, and 7A and B) (Febbraio et al. 1996, 1998). The increased muscle glycogen utilisation, muscle lactate accumulation and whole-body carbohydrate utilisation with independent alterations in circulating adrenaline and body temperature during prolonged exercise in the heat support this contention (Febbraio et al. 1996, 1998).
Heat transfer across the exercising leg
A major finding of this study was that convective heat transfer from the exercising leg to the core only represented 35-40 % of the leg metabolic heat production (Figs 5A and B, and 8C). Therefore, the present finding refutes the traditional concept that the majority of the heat produced by the active leg muscles is transferred to the core and then dissipated from the upper body skin (Nadel, 1988). Interestingly, the opposite actually occurs during the transition from rest to exercise; i.e. heat is transferred from the warmer core to the cooler exercising muscles (Fig. 9). As depicted in Fig. 8B, the femoral v-a temperature difference averaged -0.47°C during the first minute of exercise, thus leading to a net heat gain by the exercising leg of ∼8 kJ. Only after 6 min of exercise did the femoral v-a temperature difference become positive, resulting in a net heat release from the leg to the core (Figs 8B and 9). Heat transfer within the legs to the surrounding tissue can occur via skin blood convection and conduction. During exercise in a 35°C environment, skin blood convection appears to be the most important pathway since the muscle-to-skin temperature gradient available for conductive heat loss is only 4-6°C. Assuming that leg skin blood flow was 0.5 l min−1 and the arterial-to-saphenous blood temperature difference was 4°C, a rough estimation of 7 kJ min−1 of heat loss to the surrounding environment via convection is obtained. This would explain ∼40 % of the leg heat production, which added to the heat transfer to the core appeared to account for the major part of the ∼18 kJ min−1 mean leg heat production.
Figure 8. Blood and core temperature with progressive dehydration during exercise in the heat in one subject (follow-up study).
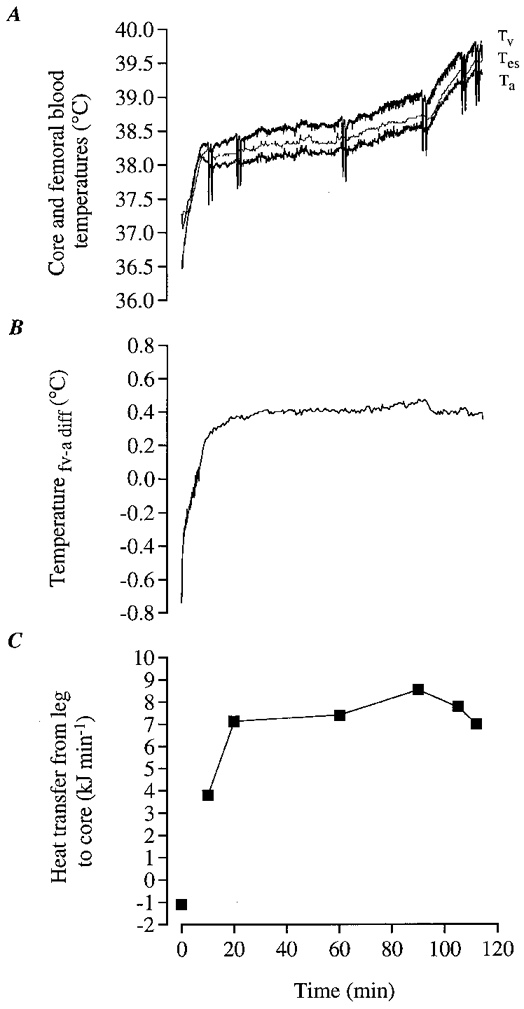
A, femoral venous blood (Tv), femoral arterial blood (Ta) and oesophageal temperature (Tes); B, femoral venous-to-arterial blood temperature difference; C, rate of heat removal from the active legs by the blood. In A, the drastic drop in venous blood temperature at several time points during exercise was due to the infusion of cold saline while measuring LBF. In C, the kinetics of heat transfer from the exercising leg to the core during the transition from rest to 10 min of exercise was omitted because LBF was not measured during this period.
Figure 9. Body temperature during the transition from rest to exercise in one subject.
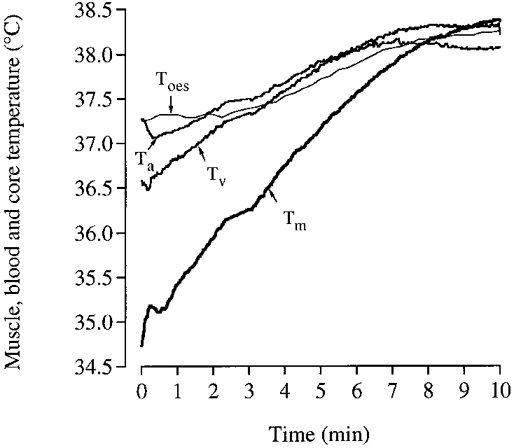
Tm, muscle (vastus lateralis); Ta, femoral arterial blood; Tv, femoral venous blood; and Toes, oesophageal temperature.
Influence of hyperthermia and altered metabolism on fatigue with dehydration
Another salient observation of this study was that fatigue with dehydration was not related to either hypoglycaemia, very low muscle glycogen or critically high muscle and circulating metabolite levels, but rather it was associated with a high body temperature (see Fig. 7A). This is based on the observations that blood glucose and muscle glycogen levels were still high (4.6-5.0 mmol l−1 and 306 mmol (kg dry weight)−1) and the absolute levels of muscle lactate as well as the between-trial differences in other metabolites proposed to be involved in fatigue such K+ and H+ were small. Instead, hyperthermia might have been the most important factor leading to fatigue with dehydration since exercise performance in the heat in these trained subjects was previously found to be closely associated with high body temperature (González-Alonso et al. 1999). In our recent parallel study, these cyclists also exercising at 60 %V̇O2,max fatigued at remarkably similar oesophageal (Toes) and muscle (Tm) temperatures (40.1-40.3°C and 40.7-40.9°C, respectively) and almost maximal heart rates (95-99 % of maximal value), regardless of the wide differences in the initial value and the rate of rise of body temperature (González-Alonso et al. 1999). Time to exhaustion ranged from 28 to 63 min, being the shortest with the highest initial body temperature and highest rate of heat storage. In the present study, fatigue coincided with a ∼0.5°C lower internal body temperature (39.7 ± 0.2 and 40.4°C in Toes and Tm, respectively), suggesting that other factors, such as altered metabolism, reduced energy provision and longer duration of exercise impaired the tolerance to hyperthermia. In agreement with these observations, dehydrated subjects have been previously shown to fatigue at a significantly lower core temperature compared with when they were euhydrated (Sawka et al. 1992).
The present findings provide new insights into a possible mechanism of fatigue in dehydrated subjects during exercise in the heat at lower body temperatures. It is apparent that the main end-result of reductions in systemic and exercising muscle blood flow with dehydration is the lowering in O2 delivery to the exercising legs at exhaustion, which in turn leads to a somewhat reduced total energy turnover (5 %; n.s.) despite the significant increase in anaerobic energy provision (see Fig. 5A). Although this hypothesis of a mismatch between energy requirement and energy turnover needs further elucidation, evidence in the literature clearly indicates that reductions in O2 delivery to the exercising muscle drastically impair oxidative energy turnover and high intensity exercise performance (Saltin et al. 1968; Knight et al. 1993). When cycling at work rates between 250 and 300 W, Knight et al. (1993) found that two-legged V̇O2 and systemic V̇O2 were ∼0.5 l min−1 and ∼0.7 l min−1 lower, respectively, with reduced O2 delivery compared with that expected under normal conditions. The present observation warrants further studies, particularly in conditions in which dehydration and hyperthermia markedly reduce maximal O2 uptake and endurance performance at constant power output.
In conclusion, the present results demonstrate that significant reductions in muscle blood flow with dehydration do not impair either the delivery of glucose and FFA to or the removal of lactate from exercising muscle during prolonged exercise. However, dehydration results in lower FFA uptake and higher muscle glycogen utilisation and lactate production by the exercising muscles, resulting in 8 % greater carbohydrate utilisation compared with control. Despite differences in substrate utilisation over time, total leg energy turnover was increased similarly (6-7 %) in both trials during the first 2 h of exercise. A major finding is that more than one-half of the metabolic heat liberated in the contracting leg muscles during prolonged exercise in the heat is dissipated directly to the environment surrounding the exercising leg. Fatigue with dehydration was not related to either critically elevated muscle lactate or critically reduced blood glucose and muscle glycogen. Instead, fatigue with dehydration appeared to be largely associated with high body temperature.
Acknowledgments
The excellent technical assistance of Ingelise Kring, Merete Vannby, Winnie Taagerund, Carsten Nielsen, Christina Teller and Signe Andersen is acknowledged. Special thanks are given to Professor Bengt Saltin for his supervision of the project and critique of the manuscript. The authors also thank Dr Ricardo Mora-Rodríguez for a critique of the manuscript. This study was supported by grants from the European Commission and Team Danmark. J. G.-A. was supported by a Marie Curie Research Training Grant (FMBICT950007).
References
- Ahlborg G, Felig P. Substrate utilisation during prolonged exercise preceded by ingestion of glucose. American Journal of Physiology. 1977;233:E188–194. doi: 10.1152/ajpendo.1977.233.3.E188. [DOI] [PubMed] [Google Scholar]
- Ahlborg G, Felig P, Hagenfeldt L, Hendler R, Wahren J. Substrate turnover during prolonged exercise in man: splanchnic and leg metabolism of glucose, free fatty acids and amino acids. Journal of Clinical Investigation. 1974;53:1080–1090. doi: 10.1172/JCI107645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. The Journal of Physiology. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsbo J, Graham T, Johansen L, Saltin B. Muscle lactate metabolism in recovery from intense exhaustive exercise: impact of light exercise. Journal of Applied Physiology. 1994;77:1890–1895. doi: 10.1152/jappl.1994.77.4.1890. [DOI] [PubMed] [Google Scholar]
- Bergman BC, Butterfield GE, Wolfel EE, Casazza GA, Lopaschuk GD, Brooks G. Evaluation of exercise and training on muscle lipid metabolism. American Journal of Physiology. 1999;276:E106–117. doi: 10.1152/ajpendo.1999.276.1.E106. [DOI] [PubMed] [Google Scholar]
- Borg G. Simple rating methods for estimation of perceived exertion. In: Borg G, editor. Physical Work and Effort. New York: Pergamon Press; 1975. pp. 39–46. [Google Scholar]
- Brooks GA, Hittleman KJ, Faulkner JA, Beyer RA. Temperature, skeletal muscle mitochondrial functions, and oxygen debt. American Journal of Physiology. 1971;220:1053–1059. doi: 10.1152/ajplegacy.1971.220.4.1053. [DOI] [PubMed] [Google Scholar]
- Christiansen NJ, Vestergaard P, Sørensen T, Rafaelsen OJ. Cerebrospinal fluid adrenaline and noradrenaline in depressed patients. Acta Psychiatrica Scandinavica. 1980;61:178–182. doi: 10.1111/j.1600-0447.1980.tb00577.x. [DOI] [PubMed] [Google Scholar]
- Coyle EF, Jeukendrup AE, Wagenmarkers AJ, Saris W. Fatty acid oxidation is directly regulated by carbohydrate metabolism during exercise. American Journal of Physiology. 1997;273:E268–275. doi: 10.1152/ajpendo.1997.273.2.E268. [DOI] [PubMed] [Google Scholar]
- Douglas A, Jones N, Reed J. Calculation of whole blood CO2 content. Journal of Applied Physiology. 1988;65:473–477. doi: 10.1152/jappl.1988.65.1.473. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Carey MF, Snow RJ, Stathis CG, Hargreaves M. Influence of elevated muscle temperature on metabolism during intense, dynamic exercise. American Journal of Physiology. 1996;271:R1251–1255. doi: 10.1152/ajpregu.1996.271.5.R1251. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Lambert DL, Starkie RL, Proietto J, Hargreaves M. Effect of epinephrine on muscle glycogenolysis during exercise in trained men. Journal of Applied Physiology. 1998;84:465–470. doi: 10.1152/jappl.1998.84.2.465. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Calbet JAL, Nielsen B. Muscle blood flow is reduced with dehydration during prolonged exercise in humans. The Journal of Physiology. 1998;513:895–905. doi: 10.1111/j.1469-7793.1998.895ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alonso J, Mora-Rodríguez R, Below PR, Coyle EF. Dehydration reduces cardiac output and increases systemic and cutaneous vascular resistance during exercise. Journal Applied Physiology. 1995;79:1487–1496. doi: 10.1152/jappl.1995.79.5.1487. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Mora-Rodríguez R, Below PR, Coyle EF. Dehydration markedly impairs cardiovascular function in hyperthermic endurance athletes during exercise. Journal of Applied Physiology. 1997;82:1229–1236. doi: 10.1152/jappl.1997.82.4.1229. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Teller C, Andersen SL, Jensen FB, Hyldig T, Nielsen B. Influence of body temperature on the development of fatigue during prolonged exercise in the heat. Journal of Applied Physiology. 1999;86:1032–1039. doi: 10.1152/jappl.1999.86.3.1032. [DOI] [PubMed] [Google Scholar]
- Graham TE, Kiens B, Hargreaves M, Richter E. Influence of fatty acids on ammonia acid flux from active human muscle. American Journal of Physiology. 1991;261:E168–176. doi: 10.1152/ajpendo.1991.261.2.E168. [DOI] [PubMed] [Google Scholar]
- Hamilton MT, González-Alonso J, Montain SJ, Coyle EF. Fluid replacement and glucose infusion during exercise prevent cardiovascular drift. Journal of Applied Physiology. 1991;71:871–877. doi: 10.1152/jappl.1991.71.3.871. [DOI] [PubMed] [Google Scholar]
- Hargreaves M, Angus D, Howlett K, Conus NM, Febbraio M. Effect of heat stress on glucose kinetics during exercise. Journal of Applied Physiology. 1996a;81:1594–1597. doi: 10.1152/jappl.1996.81.4.1594. [DOI] [PubMed] [Google Scholar]
- Hargreaves M, Dillo P, Angus D, Febbraio M. Effect of fluid ingestion on muscle metabolism during prolonged exercise. Journal of Applied Physiology. 1996b;80:363–366. doi: 10.1152/jappl.1996.80.1.363. [DOI] [PubMed] [Google Scholar]
- Kiens B, Essen-Gustavsson B, Christensen NJ, Saltin B. Skeletal muscle substrate utilisation during submaximal exercise in man: effect of endurance training. The Journal of Physiology. 1993;469:459–478. doi: 10.1113/jphysiol.1993.sp019823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjær M, Engfred K, Fernandes A, Secher NH, Galbo H. Regulation of glucose production during exercise in humans: role of sympathoadrenergic activity. American Journal of Physiology. 1993;265:E275–283. doi: 10.1152/ajpendo.1993.265.2.E275. [DOI] [PubMed] [Google Scholar]
- Kjær M, Kiens B, Hargreaves M, Ritcher E. Influence of active muscle mass on glucose homeostasis during exercise in humans. Journal of Applied Physiology. 1991;71:552–557. doi: 10.1152/jappl.1991.71.2.552. [DOI] [PubMed] [Google Scholar]
- Knight DR, Schaffartizik W, Poole DC, Hogan MC, Bebout DE, Wagner P. Effects of hyperoxia on maximal leg O2 supply and utilisation in man. Journal of Applied Physiology. 1993;75:2586–2594. doi: 10.1152/jappl.1993.75.6.2586. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV. A Flexible System of Enzymatic Analysis. New York: Academic Press; 1972. pp. 199–201. [Google Scholar]
- Montain SJ, Coyle EF. Fluid ingestion during exercise increases skin blood flow independent of increases in blood volume. Journal of Applied Physiology. 1992a;73:903–910. doi: 10.1152/jappl.1992.73.3.903. [DOI] [PubMed] [Google Scholar]
- Montain SJ, Coyle EF. The influence of graded dehydration on hyperthermia and cardiovascular drift during exercise. Journal of Applied Physiology. 1992b;73:1340–1350. doi: 10.1152/jappl.1992.73.4.1340. [DOI] [PubMed] [Google Scholar]
- Montain SJ, Hopper MK, Coggan AR, Coyle EF. Exercise metabolism at different time intervals after a meal. Journal of Applied Physiology. 1991;70:882–888. doi: 10.1152/jappl.1991.70.2.882. [DOI] [PubMed] [Google Scholar]
- Montain SJ, Smith SA, Mattot RP, Zientara GP, Jolesz FA, Sawka MN. Hypohydration effects on skeletal muscle performance and metabolism: a 31P-MRS study. Journal of Applied Physiology. 1998;84:1889–1894. doi: 10.1152/jappl.1998.84.6.1889. [DOI] [PubMed] [Google Scholar]
- Nadel ER. Temperature regulation and prolonged exercise. In: Lamb DR, Murra YR, editors. Perspectives in Exercise Science and Sports Medicine, Prolonged Exercise. I. Indianapolis, Indiana: Benchmark Press; 1988. pp. 125–151. [Google Scholar]
- Nielsen B, Hales JRS, Strange S, Christiensen NJ, Warberg J, Saltin B. Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. The Journal of Physiology. 1993;460:467–485. doi: 10.1113/jphysiol.1993.sp019482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen B, Kubica R, Bonnesen A, Rasmussen IB, Stoklosa J, Wilk B. Physical work capacity after dehydration and hyperthermia. Scandinavian Journal of Sports Science. 1981;3:2–10. [Google Scholar]
- Nielsen B, Savard G, Richter EA, Hargreaves M, Saltin B. Muscle blood flow and metabolism during exercise and heat stress. Journal of Applied Physiology. 1990;69:1040–1046. doi: 10.1152/jappl.1990.69.3.1040. [DOI] [PubMed] [Google Scholar]
- Péronnet F, Massicotte D. Table of nonprotein respiratory quotient: an update. Canadian Journal of Sports Science. 1991;16:23–29. [PubMed] [Google Scholar]
- Pitts GC, Johnson RE, Consolazio FC. Work in the heat as affected by intake of water, salt and glucose. American Journal of Physiology. 1944;142:253–259. [Google Scholar]
- Richardson RS, Noyszewski EA, Kendrick KF, Leigh JS, Wagner PD. Myoglobin O2 desaturation during exercise: Evidence of limited O2 transport. Journal of Clinical Investigation. 1995;96:1916–1926. doi: 10.1172/JCI118237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. American Journal of Physiology. 1993;265:E380–391. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- Sahlin K, Tonkonogi M, Söderlund K. Energy supply and muscle fatigue in humans. Acta Physiologica Scandinavica. 1998;162:261–266. doi: 10.1046/j.1365-201X.1998.0298f.x. [DOI] [PubMed] [Google Scholar]
- Saltin B. Aerobic and anaerobic work capacity after dehydration. Journal of Applied Physiology. 1964;19:1114–1118. doi: 10.1152/jappl.1964.19.6.1114. [DOI] [PubMed] [Google Scholar]
- Saltin B, Grover RF, Bromqvist CG, Hartley LH, Johnson RL. Maximal oxygen uptake and cardiac output after 2 weeks at 4,300 m. Journal of Applied Physiology. 1968;25:400–409. [Google Scholar]
- Sawka MN, Knowlton RG, Critz JB. Thermal and circulatory responses to repeated bouts of prolonged running. Journal of Applied Physiology. 1979;19:833–838. [PubMed] [Google Scholar]
- Sawka MN, Young AJ, Latzka WA, Neufer PD, Quicley MD, Pandolf KB. Human tolerance to heat strain during exercise: influence of hydration. Journal of Applied Physiology. 1992;50:772–778. doi: 10.1152/jappl.1992.73.1.368. [DOI] [PubMed] [Google Scholar]
- Wilson DL, Erecinska M, Drown C, Silver IA. Effect of oxygen tension on cellular energetics. American Journal of Physiology. 1977;233:C135–140. doi: 10.1152/ajpcell.1977.233.5.C135. [DOI] [PubMed] [Google Scholar]


