Figure 1. Morphology and electrophysiology of stable π-junctions.
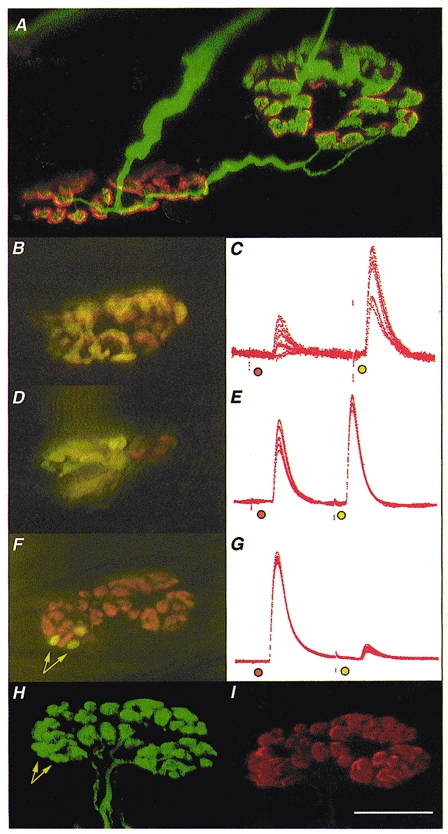
Immunocytochemistry was used to visualise nerve terminals (FITC) and TRITC-α-BTX was used to visualise AChRs in fixed preparations; FM1-43 and RH414 were used to label synaptic boutons supplied by SN and LPN motor axons in freshly isolated preparations, staining their recycled synaptic vesicles fluorescent green/yellow and orange, respectively. A, confocal microscope image of two motor endplates in a reinnervated muscle, 9 weeks after LPN crush (including 4 weeks recovery from a 2 week nerve conduction block, applied 3 weeks after the original crush). The endplates are bridged by an axonal sprout, probably arising from the motor nerve terminal on the left. The endplate on the right is also innervated by a slender regenerating axon. Thus, this endplate is a stable π-junction. B, D and F, composite digital images from three different, vitally stained stable π-junctions innervated by LPN (orange, RH414 labelled) and SN (yellow/green, FM1-43 labelled) terminal boutons, several weeks after regeneration and recovery from nerve conduction block. The image in F is a montage made up from two original digital images taken in slightly different focal planes. C, E and G, corresponding intracellular recordings of EPPs evoked from these π-junctions after bathing the preparations in μ-conotoxin to abolish muscle (but not axonal) action potentials. In each case the orange spot indicates LPN stimulation and the yellow/green spot indicates SN stimulation. The relative EPP amplitudes varied in proportion to the relative areas covered by LPN and SN synaptic boutons. The π-junction shown in F was relocated using a confocal microscope after fixing and staining for neurofilament/SV2 (H; FITC-conjugated secondary antibody, green fluorescence) and ACh receptors (I; TRITC-α-BTX, red fluorescence). Data from this endplate were as follows: in response to stimulating the nerve supplies repeatedly at 1 Hz, the LPN produced a large amplitude EPP (40 ± 0.167 mV, n = 110; uncorrected amplitudes; 93 % of the total synaptic response; mean quantal content, 47.4) and the SN produced a proportionally smaller amplitude EPP (2.9 ± 0.168 mV; 7 % of the total synaptic response; 13.8 times smaller than LPN response; mean quantal content, 3.1). The synaptic area covered by the LPN was 137 μm2 (93.2 % of total area) and the SN covered 10 μm2 (arrows in F; 6.8 % of total area; 13.7 times smaller than LPN). The confocal images confirmed that the differently coloured boutons were supplied by distinct axons directed to the same endplate, and that all boutons visualised immunocytochemically were also stained with the vital dyes before fixation. Arrows in H point to the location of the same boutons as indicated in F. There was no discernible difference in the fluorescence intensity of receptors in the region of the endplate occupied by the three SN boutons compared with receptors occupied by LPN boutons. Calibrations: scale bar in I corresponds to the following measures: A, 10 μm; B, D and F, 20 μm; H and I, 15 μm; C, E and G, 25 ms. The electrophysiological records were scaled to match the largest EPP in each case. These were as follows: C, 6 mV; E, 24 mV; G, 42 mV.
