Figure 1. Regulation of myosin II in smooth and non-muscle cells.
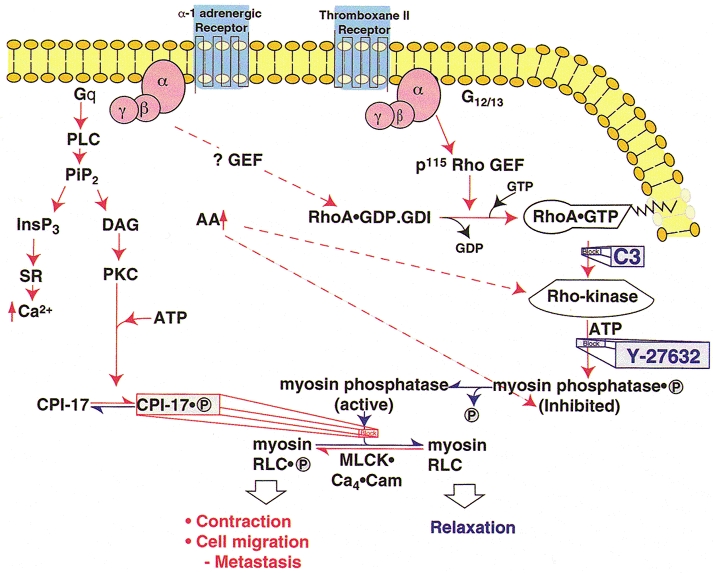
Pathways indicated in red activate myosin II, resulting in contraction, cell migration and cancer metastasis. Pathways that reduce myosin II activity are shown in blue. The major, Ca2+-independent pathway that increases myosin II activity is through activation of Rho-kinase by RhoA.GTP, phosphorylation of the regulatory subunit of myosin phosphatase (MLCP) by Rho-kinase and some other kinases (see text), resulting in inhibition of MLCP activity and increased myosin RLC phosphorylation. Increases in arachidonic acid, due to a variety of stimuli, can also activate Rho-kinase and, at least in vitro, also inhibit MLCP activity by dissociating the regulatory (M110-130) from the catalytic (PP-1C) subunit. The third pathway shown that enhances myosin II activity is through phosphorylation of CPI-17 by protein kinase C (PKC) leading to direct inhibition of PP-1C by the phosphorylated CPI-17 (CPI-17.P). Each of these mechanisms requires the presence of an active kinase that can phosphorylate Ser19 of RLC and increase myosin II activity by inhibiting MLCP even at constant [Ca2+]i (‘Ca2+ sensitization’). These mechanisms operate in parallel with and independently of the activation of MLCK by Ca2+ released from the sarcoplasmic reticulum/endoplasmic reticulum (SR/ER) by InsP3 or by Ca2+ influx (not shown). Abbreviations: PLC, phospholipase C; PiP2, phosphatidylinositol-bis-phosphate; InsP3, inositol 1,4,5-trisphosphate; DAG, diacylglycerol; PKC, protein kinase C; GEF, guanine nucleotide exchange factor; MLCK, myosin light chain kinase; Cam, calmodulin; RLC, regulatory light chain; C3, Clostridium botulinum, exoenzyme that ADP-ribosylates Asn 41 of RhoA, inhibits RhoA activity and blocks its translocation to the membrane; Y-27632, selective inhibitor of Rho-kinase.
