Figure 11. Model of [K+]o regulation in rat optic nerve.
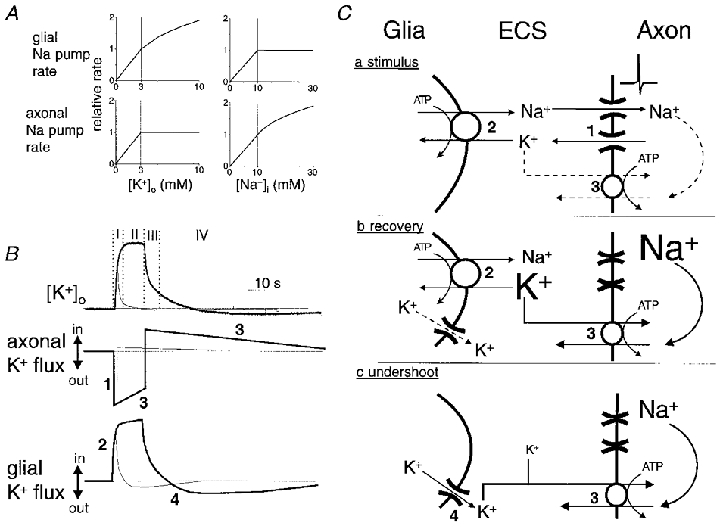
A, graphical illustration of two assumptions of our model. We assume that the rate of glial Na+,K+-ATPases, but not that of axonal Na+,K+-ATPases, is directly related to [K+]o over the range of concentrations encountered in our experiments. The rate of glial pumps adjusts instantaneously to changes in [K+]o. The external K+-binding site of axonal Na+ pumps is maximally stimulated when [K+]o is 3 mM. We assume that the rate of axonal Na+,K+-ATPases is sensitive to elevations of [Na+]i above the resting value of ≈10 mM. The rate of glial Na+ pumps falls off with reductions in [Na+]i. B, hypothetical relationship of transmembrane K+ fluxes in axons and glia to activity-dependent changes in [K+]o. Thick lines represent the situation following a 10 s stimulus; thin lines represent the situation following a 1 s stimulus. Immediately following the onset of stimulation there is an efflux of K+ from axons (mechanism 1 in C). Glial pumps instantaneously adjust their rate to the new [K+]o (mechanism 2 in C). Net efflux of K+ from axons drops slowly during the period of stimulation as Na+ loading of the intra-axonal volume enhances the rate of K+ influx via the axonal Na+ pumps (mechanism 3 in C). Following cessation of electrical stimulation axons immediately switch from a net efflux to a net influx of K+ as axons repolarise and K+ channels inactivate while their Na+ pumps continue at an accelerated rate to extrude accumulated sodium ions. Glial Na+ pump rate falls quickly as [K+]o decays. During the undershoot of [K+]o glia bleed the potassium ions that they transiently accumulated back to the ECS, probably via ion channels (mechanism 4 in C), for recapture by the axonal Na+ pumps that continue to operate above basal rates. When [K+]o returns to baseline, axons and glia have restored their initial intracellular concentrations, i.e. the area of the efflux portion of these curves equals the area of the influx portion. In summary, (I) mechanisms 1 and 2 determine [K+]o immediately following onset of stimulation and mechanism 2 is mainly responsible for determining the magnitude of evoked [K+]o increases following short duration stimuli (< 1 s), (II) mechanisms 1, 2 and 3 determine [K+]o during sustained stimulation with an increased role for mechanism 3 as the stimulus is made longer, (III) mechanisms 2 and 3 contribute to the rapid fall of [K+]o following cessation of stimulation (with mechanism 2 predominating for short periods of stimulation), and (IV) mechanisms 3 and 4 determine [K+]o during the undershoot. This model implies that glia are the primary regulators of the magnitude of evoked [K+]o increases, especially following short duration stimuli. Likewise, glial pumps play a relatively larger role in post-stimulus recovery of [K+]o increases following short duration stimuli. C, schematic diagram of the hypothesised mechanisms determining the [K+]o during electrical stimulation (a), post-stimulus recovery (b), and the undershoot in [K+]o (c).
